



In order to start to think about making calculations, it is important to familiarize yourself with reference tools that are available. The following link will connect you with the Chemistry reference tool for the STAAR chemistry exam.
Answer the following questions in your notes. After attempting to answer the questions, self-check your answers below.




The ideal gas law is represented using the following equation:
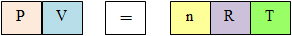
However, when you are asked to calculate information using an equation, more often than not, you will have to manipulate (or change) the equation to find what you need. Remember when solving for a particular item, the goal is to get the item alone on one side of the "equals" sign.
Using the cards provided, take a few moments to see if you can come up with the correct equation for the item you are solving for. Drag the cards given into the final formula section in the order that you think they would represent the new modified formula. When you get the cards in the correct order, the correct equation will appear in the answer box.
Scenario 1: You have been given the following information: pressure, volume, moles (n), and the constant (R). What equation would you use to find the temperature?
![]() Click here to begin the activity.
Click here to begin the activity.
Scenario 2: You have been given the following information: volume, moles (n), temperature, and the constant (R). What equation would you use to find the pressure?
![]() Click here to begin the activity.
Click here to begin the activity.
Scenario 3: You have been given the following information: pressure, moles (n), temperature, and the constant (R). What equation would you use to find the volume?
![]() Click here to begin the activity.
Click here to begin the activity.
Scenario 4: You have been given the following information: volume, temperature, pressure, and the constant (R). What equation would you use to find the moles (n)?
![]() Click here to begin the activity.
Click here to begin the activity.
When you have completed the above activity, click below to see the reference table. Interactive popup. Assistance may be required.
| Original Ideal Gas Law Equation | (PV) = (nRT) |
| Solving for Temperature | T = (PV)/(nR) |
| Solving for Pressure | P = (nRT)/V |
| Solving for Volume | V = (nRT)/P |
| Solving for Moles (n) | n = (PV)/(RT) |
