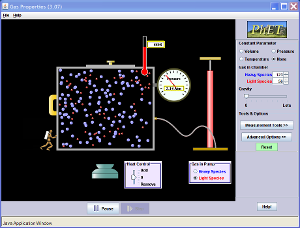
The ideal gas law is a combined set of gas laws that is a thermodynamic equation that allows us to relate the temperature, volume, and number of molecules (or moles) present in a sample of a gas. The ideal gas law was discovered by physicist and engineer Benoît Paul Émile Clapeyron (seen on the right) in 1834. You may have seen the equation PV = nRT in your classes before. This is the ideal gas law equation, and it is the use of this equation that helps us understand and control the behavior of gases we use in everyday objects such as soda cans, scuba tanks, weather balloons, and car airbags.

Source: Benoit Clapeyron, Ahellwig, Wikimedia Commons