
Where does the number 6.02 × 1023 come from? Did chemists just pull that number out of thin air? Of course not! The isotope carbon 12 has 6.02 × 1023 carbon atoms in a 12 gram sample. So, 12 grams of carbon minus 12 equals one mole of carbon-12. The number of atoms in 12 grams of carbon-12, or 6.02 × 1023, was set as Avogadro’s number.
Let’s think about Avogadro's number in terms of the macroscopic world.

If you had a mole of pennies, you would have 6.02 × 1023 pennies. That is a lot of pennies. If a single mole of pennies were divided among all the living people in the world, each person could spend a million dollars per hour for the rest of his or her life.
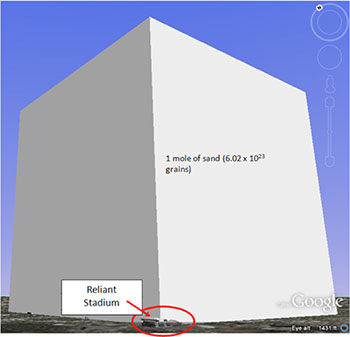
Now imagine a mole of sand particles. That would be 6.02 x 1023 grains of sand. How big would it be? If you put all of that sand in a cube shaped box, it would be about 4,400 meters tall, 4,400 meters wide and 4,400 meters deep. It would dwarf the Astrodome and Reliant Stadium in Houston (to the left).
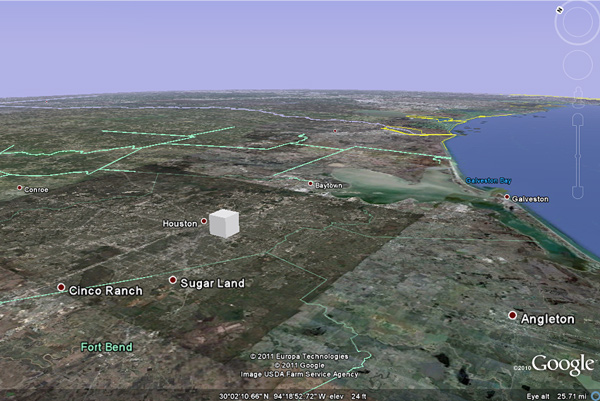
The image below shows what the mole of sand would look like if you were flying from west to east toward Houston.
Fortunately, you only use moles to measure amounts of particles such as ions, atoms, molecules, and formula units. These particles are so small that a mole of sulfur, oxygen, sodium chloride, or other substance is a reasonable amount.

Earlier in this section, it was stated that 12 grams of carbon-12 equals one mole of carbon-12.

One mole of copper is equal to 63 grams of copper.

One mole of sulfur is equal to 32.06 grams of sulfur.
Look at the partial periodic table pictured below.
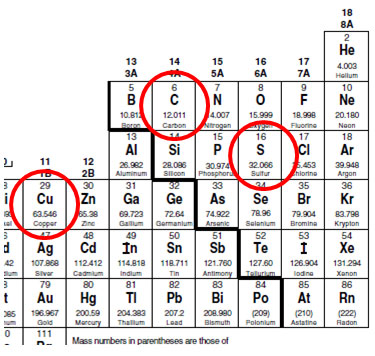
What do you notice about the number of grams in one mole of the elements listed above and the atomic masses of the elements?
Interactive popup. Assistance may be required.
They are the same.

For any element, a mole is the atomic mass expressed in grams.
What about compounds such as glucose, C6H12O6? How much is one mole of glucose?
One mole of glucose is 180 grams.
For any compound, a mole is the formula, or molecular mass, in grams. We can use a table like the one below to determine the total mass of glucose.
| Element | Atomic Mass (from the periodic table) | Multiplied by | # of atoms present in the compound | total mass of each element present in the compound |
| Carbon | 12.0 |
X |
6 |
72.0 grams |
| Hydrogen | 1.0 |
X |
12 |
12.0 grams |
| Oxygen | 16.0 |
X |
6 |
96.0 grams |
Total Mass |
180.0 grams | |||
What about a mole of a gaseous element or compound? How do we measure a mole of oxygen, for example? Fortunately, you can use volume to measure moles of gas. In studying the properties of gases, Amadeo Avogadro discovered that equal volumes of different gases at the same temperature and pressure have the same number of particles.
One mole of any gas at a standard temperature and pressure has a volume of 22.4 liters.
Why do chemists use the mole?

Let’s say you are conducting a laboratory exercise where you are required to measure one mole of sulfur. Could you count out 6.02 × 1023 atoms of sulfur? No, you could not, but you could mass out 32.06 grams of sulfur. You now know that 32.06 grams of sulfur would equal one mole!
Sources of images used for this section as they appear, top to bottom: