
When you hear the phrases a dozen roses, a dozen eggs, or a dozen doughnuts, what number do you think of?

You think of the number 12, right? A dozen is a way of counting items. It is not a way of expressing weight or mass. After all, the items of 12 pictured above do not all have the same mass or weigh the same amount, do they?
In chemistry, a mole is a way of counting. So, if the word dozen stands for the number 12, what does the word mole stand for? The word mole stands for the number
602,200,000,000,000,000,000,000
That is an extremely large number! So chemists use scientific notation, or 6.02 × 1023, when writing the number. 6.02 × 1023 is commonly called Avogadro's number after Amedeo Avogadro, the scientist who laid the groundwork for the principle of the mole.
Why do chemists use such a large number to count? A mole stands for a certain number of things. Normally, the things counted in a mole are extremely small or microscopic. These things can use particles such as:
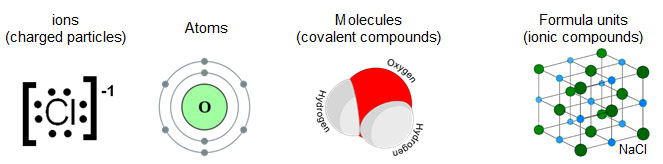
It takes to a large amount (6.02 × 1023) of the particles to have an amount that chemist can work with in the lab.
Just as the dozen donuts, dozen flowers, and dozen eggs did not all have the same mass or weigh the same, a mole of any particle will not have the same mass or weigh the same as a mole of another particle. For example, a mole of iron will not have the same mass as a mole of copper or a mole of tin or a mole of iodine. The picture below shows one mole of atoms of iron, copper, tin, and iodine.

Sources of images used for this section as they appear, top to bottom: