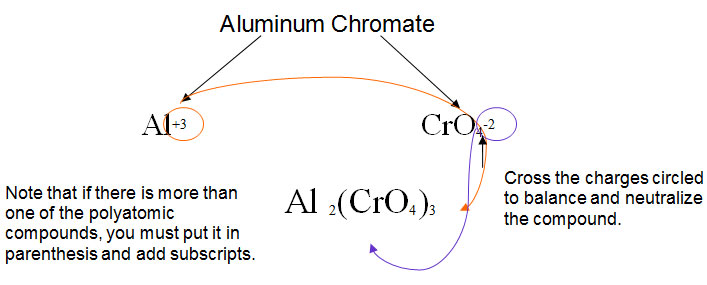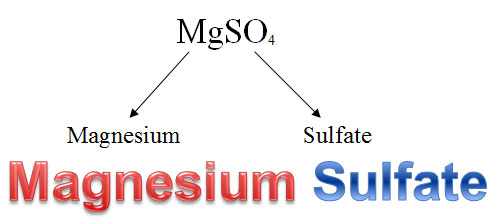
Naming compounds with polyatomic ions is very similar to naming binary compounds, but you must use your STAAR reference material to determine the proper name and formula for the polyatomic portion of the compound. For example, Mg could bond with SO4-2, sulfate, to make MgSO4, magnesium sulfate. When naming ionic compounds involving polyatomic ions, do not change the ending of the polyatomic anion.

The image below shows the polyatomic ions on the STAAR reference material.

![]() Watch this video, then perform the practice below.
Watch this video, then perform the practice below.
Source: Naming Compounds Containing Polyatomic Ions, kentchemistry, YouTube
![]() Practice naming the following compounds. Type the name of the compound.
Practice naming the following compounds. Type the name of the compound.
When canceling out the charges in the crisscross method, remember that polyatomic ions are treated as whole compounds.