Source: Binary Ionic compound, Preparatory Chemistry
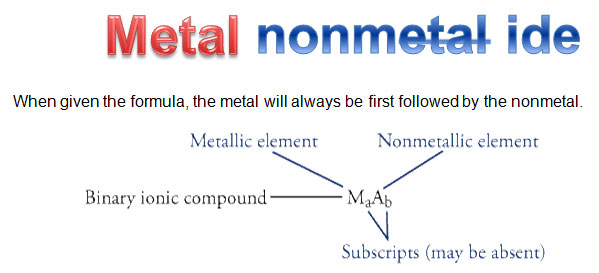
The simplest type of ionic compounds is one that involves one cation and one anion. These are referred to as a binary ionic compound. Binary means two.
The nomenclature of binary ionic compounds is straightforward. The cation, or metal, is listed first, and the anion root name is listed second. The end of the anion root name is changed to "ide." See the examples below.

Source: Binary Ionic compound, Preparatory Chemistry
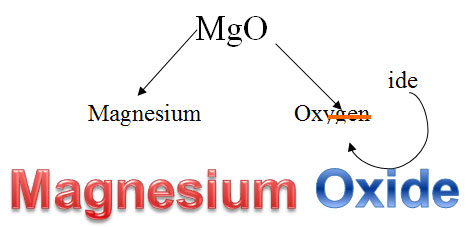
For the compound MgO, you simply name the metal, magnesium, and then look at the anion, oxygen. Use only the root –ox, and change the end to –ide to get the name magnesium oxide.
![]() Watch the following video to see examples of naming binary ionic compounds. (Note, they do not take into account the varying charges or transition metals, which will be covered in another section of this resource.)
Watch the following video to see examples of naming binary ionic compounds. (Note, they do not take into account the varying charges or transition metals, which will be covered in another section of this resource.)
Source: Naming Binary Ionic Compounds, kentchemistry, YouTube
Writing the formulas of ionic compounds is not as straightforward as naming the compound, but it is somewhat simple as long as you have a periodic table and know how to determine the oxidation number and charge of any ion.
Oxidation numbers are the resulting charge after an atom has given away or taken in electrons in order to have a full valence shell.
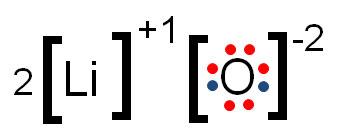
In the example above, oxygen gained two electrons to fill its shell, giving it an oxidation number of -2. Lithium lost one electron; therefore, its charge is +1, which is also its oxidation number.
The image below shows the oxidation numbers written on the periodic table found in the STAAR Reference Materials. Remember, transition metals can have different oxidation numbers. You will learn more about naming compounds involving transition metals in another section of this resource.
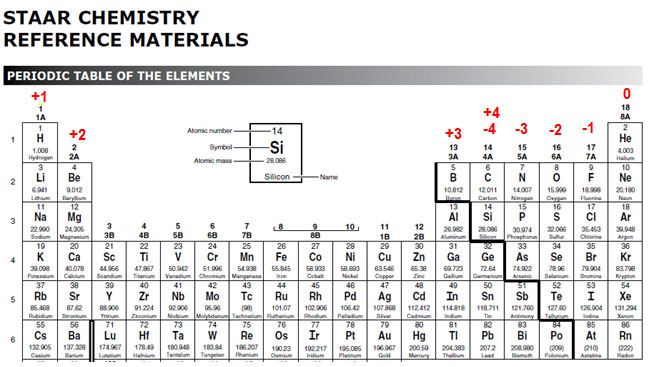
Source: STAAR Reference Material, Texas Education Agency
Now that you have reviewed how to determine an oxidation number, you can start the crisscross method. In any ionic compound, the elements that are combined in a ratio to fill each other’s valence shells end up combining in a neutrally charged compound.
![]() Let’s look at a few examples.
Let’s look at a few examples.
![]() Use this interactive activity to practice formula writing.
Use this interactive activity to practice formula writing.