Molar mass is the mass of one mole of a substance, usually expressed in grams or kilograms. Let’s say you are conducting a laboratory exercise where you are required to measure 1 mole of sulfur. Can you see yourself counting out 6.02 x 1023 atoms? If I have 1 mole of Iron, I would have 6.02 x 1023 atoms. What would be the mass of 1 mole of Iron?
Since iron has an average atomic mass from the Periodic Table of 55.8 amu, the mass of 1 mole of iron would be 55.8 grams.
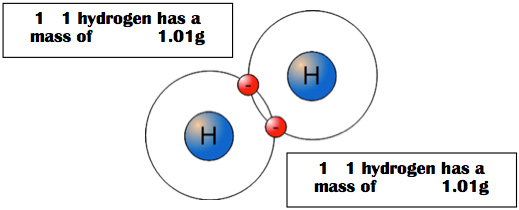
What happens with diatomic molecules like hydrogen?
1 mole of H2 is still 6.02 x 1023 particles (in this case molecules) because they are joined by shared electrons.

One mole of H2 would have a mass of 2 x 1.01g or 2.02 g/mole H2 because mass is conserved.
The molar mass of a compound is found by taking the sum of the average atomic mass of each element times its subscript.
Example
1 mole of sodium carbonate (Na2CO3) would be:
2(22.99g) + 1(12.01g) + 3(15.99g) = 105.96 grams
Why?
Why do we need to know molar mass? What if you have a laboratory exercise which calls for 1 mole of manganese. You couldn't very well count out 602,000,000,000,000,000,000,000 (6.02 x1023) atoms could you? But what you can do is measure out the amount of manganese, in grams, that will give you 6.02 x 1023.