 Source: Periodic Table, Luigi Chiesa, Wikimedia Commons, http://upload.wikimedia.org/wikipedia/commons/e/e7/Periodic_table-metals.svg
Source: Periodic Table, Luigi Chiesa, Wikimedia Commons, http://upload.wikimedia.org/wikipedia/commons/e/e7/Periodic_table-metals.svg
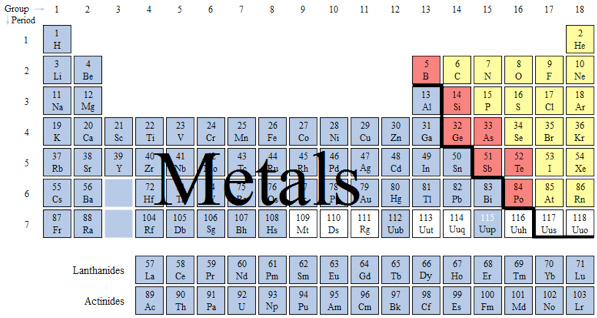
A metal is an element or compound with high electrical conductivity, usually has a shiny surface, can be melted, hammered into sheets and or drawn into wires. In a metal, atoms lose electrons easily and form positive ions (cations). Those ions are surrounded by electrons, which “roam” about the metal structure. The “roaming” electrons are called delocalized electrons.
Metals occupy the majority of the periodic table.
 Source: Periodic Table, Luigi Chiesa, Wikimedia Commons, http://upload.wikimedia.org/wikipedia/commons/e/e7/Periodic_table-metals.svg
Source: Periodic Table, Luigi Chiesa, Wikimedia Commons, http://upload.wikimedia.org/wikipedia/commons/e/e7/Periodic_table-metals.svg
The images below show various elemental metals. Click on the image below to go to the Photographic Periodic Table. Then click on the different metal elements to see photos and details about those metals.