Interactive popup. Assistance may be required.

Interactive popup. Assistance may be required.

Interactive popup. Assistance may be required.

Interactive popup. Assistance may be required.


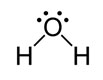
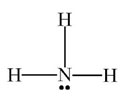
Now try these with double or triple bonds.
Interactive popup. Assistance may be required.
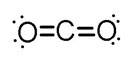

Interactive popup. Assistance may be required.
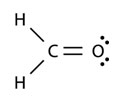

Interactive popup. Assistance may be required.
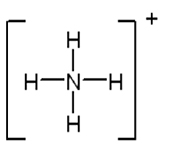

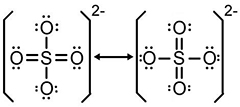
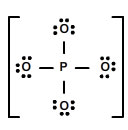
Now try drawing the Lewis structures for these molecules. Include all resonance structures and formal charges.
Interactive popup. Assistance may be required.
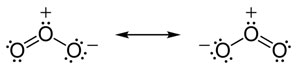

Interactive popup. Assistance may be required.
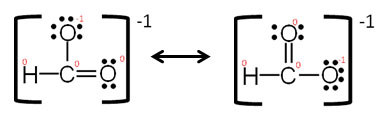

Interactive popup. Assistance may be required.