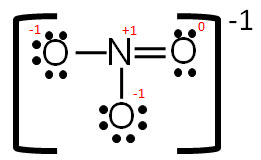
Let’s look back at the electron dot structure you drew for nitrate, NO3-1.

What if the double bond was placed between one of the other oxygen atoms and the nitrogen? Would the structure be correct?
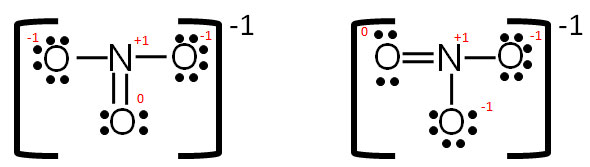
These are called resonance structures. Resonance structures are represented with a double-headed arrow. In this case, nitrate has three resonance structures.
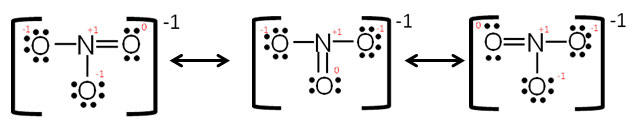
You must show as many resonance structures as it takes to represent a version where each atom able to double bond is shown doing so. The double bond is only interchangeable with other atoms of the same element bonded to the same central atom.
![]() Watch the following video to better understand this concept.
Watch the following video to better understand this concept.
Source: Drawing Lewis Structures, Resonance structures-chemistry tutorial, The Chemistry solution, YouTube