![]() Watch the following video summarizing the concepts you learned with illustrations and a step-by-step approach.
Watch the following video summarizing the concepts you learned with illustrations and a step-by-step approach.
Source: Limiting Reactants and Percent Yield, Bozeman Science, YouTube
Practice incorporating percent yield and limiting reactants in the same problem when solving the next two problems. Remember, the theoretical yield is always the maximum yield that can be produced by the limiting reactant.
When 10.0 grams of oxygen reacted with 0.250 moles of methane, 5.10 grams of carbon dioxide were captured. Calculate the percent yield of this reaction.
CH4 + 2 O2 → CO2 + 2 H2O
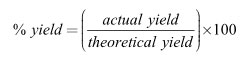

Under certain conditions the reaction between 315 grams of nitrogen and 125 grams of hydrogen produces a 43% yield. How many grams of ammonia are made in this reaction?
N2 + 3 H2 → 2 NH3