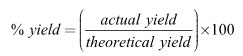
Now that you have had a little review on how percentages work, let’s look at the formula again, but this time with more specific language.
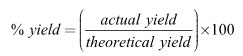
Is it possible for a percent yield in a laboratory to be greater than 100%? The answer is yes, and no. It is impossible to actually produce a greater amount of product than what stoichiometry tells you is “theoretically” possible. It is completely possible to weigh out a greater mass of what you think is “product” in a lab and record that value in your data table.
When chemical reactions are produced in a laboratory, the amount of “actual yield” is affected by the physical methods used. Even with careful attention to procedures, scientists still end up with percent yields not equal to zero. There are many reasons that this might occur.
![]() Look at the chart below. It is separated into two categories. Click on the correct column for each source of error.
Look at the chart below. It is separated into two categories. Click on the correct column for each source of error.