The three basic parts of any stoichiometry problem are as follows:

By doing stoichiometry, you are showing that both the number of particles/moles and the molar ratio between reagent and product are important. Here is the final answer to the problem you solved in the previous section.
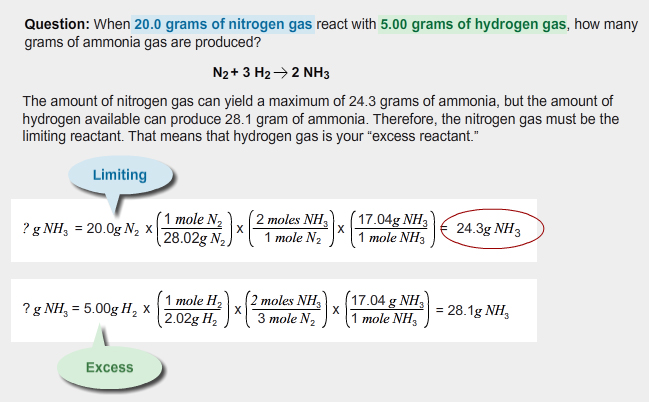
When 20.0 grams of N2 react with 5.00 grams of H2, you get 24.3 grams of NH3. That doesn’t seem to obey the law of conservation of mass! How can 25.0 grams of reagents go in, and only 24.3 grams of product come out?

Based on your calculations, fully reacting all 5.00 grams of hydrogen would have yielded, or produced, 28.1 grams of ammonia. Instead, the amount of nitrogen you had available limited you to making only 24.3 grams of ammonia. The fraction of ammonia produced must equal the fraction of excess reagent used. Here’s what that looks like mathematically.
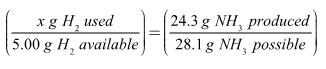
Solve for x, which represents the mass of hydrogen consumed in the reaction.
Based on your answer, how much hydrogen reagent was left over at the end of the complete reaction?

The law of conservation is always obeyed. What you just learned is that when 20.0 grams of nitrogen fully reacts with 5.00 grams of hydrogen, you get 24.3 grams of ammonia and 0.7 grams of excess hydrogen leftover. All the mass is accounted for!
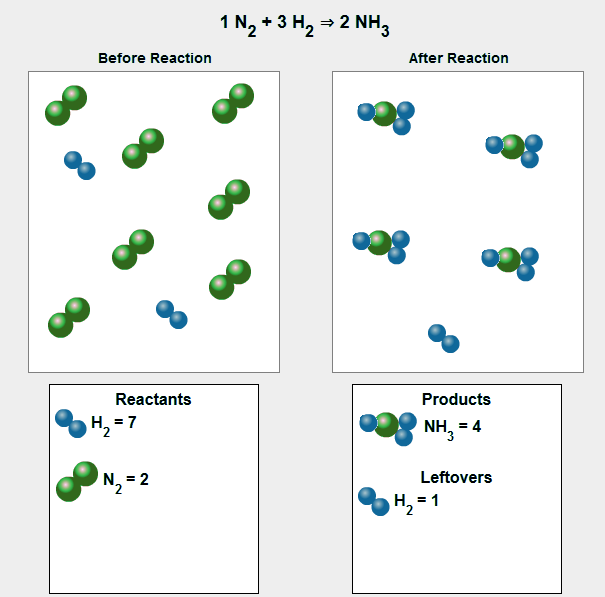
It is important to realize that ammonia molecules and hydrogen molecules are both present at the end of the reaction, but no nitrogen molecules remain. If you were looking at a particulate level, it might look something like the following: