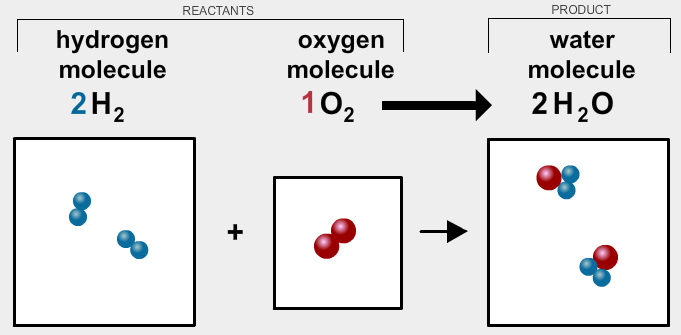
To better understand what a limiting reagent is it’s always helpful to start with a reaction you are already familiar with. Let’s revisit the formation of water from hydrogen gas and oxygen gas.

In the drawings above, you see the result of when two hydrogen molecules react with one oxygen molecule to form two water molecules.
![]() In the box on the left, you are shown four molecules of hydrogen gas with a different number of oxygen molecules. Into the empty box on the right, drag as many of each type of particle in order to represent what would be present after the reaction was complete. The law of conservation of mass must be fulfilled so that you have as many atoms of each element “Before the Reaction” as “After the Reaction.”
In the box on the left, you are shown four molecules of hydrogen gas with a different number of oxygen molecules. Into the empty box on the right, drag as many of each type of particle in order to represent what would be present after the reaction was complete. The law of conservation of mass must be fulfilled so that you have as many atoms of each element “Before the Reaction” as “After the Reaction.”
Answer the following questions about the above reaction:
Interactive popup. Assistance may be required.
Hydrogen; there were four hydrogen molecules and only one oxygen molecule.
Interactive popup. Assistance may be required.
Hydrogen; there were two leftover molecules of hydrogen when the reaction finished.
Interactive popup. Assistance may be required.
Oxygen; there was only enough oxygen to make two molecules of water. When the oxygen was used up, the excess hydrogen molecules had nothing to react with.
![]() Into the empty box on the right drag as many of each type of particle in order to represent what would be present after the reaction was complete.
Into the empty box on the right drag as many of each type of particle in order to represent what would be present after the reaction was complete.
Answer the following questions about the above reaction:
Interactive popup. Assistance may be required.
Hydrogen; there were four hydrogen molecules and only two oxygen molecules.
Interactive popup. Assistance may be required.
Neither molecule had leftovers after the reaction; both reagents were completely used up by the reaction.
Interactive popup. Assistance may be required.
Both, or either, hydrogen and oxygen limited the reaction. Even though you started with more hydrogen, no reagent molecules were leftover.
![]() Into the empty box on the right drag as many of each type of particle in order to represent what would be present after the reaction was complete.
Into the empty box on the right drag as many of each type of particle in order to represent what would be present after the reaction was complete.
Interactive popup. Assistance may be required.
Hydrogen; there were four hydrogen molecules and only three oxygen molecules.
Interactive popup. Assistance may be required.
Oxygen; there was one leftover oxygen molecule after the reaction.
Interactive popup. Assistance may be required.
Hydrogen; even though you started with more hydrogen than oxygen, you only had enough hydrogen to react with two oxygen molecules.
![]() Click on the blue arrow to see what you have learned.
Click on the blue arrow to see what you have learned.