Interactive popup. Assistance may be required.
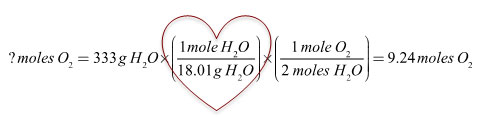
In this problem, you had to do a conversion for Phase 1, but since you wanted moles of oxygen gas, Phase 3 did not require an extra step in dimensional analysis.

Interactive popup. Assistance may be required.
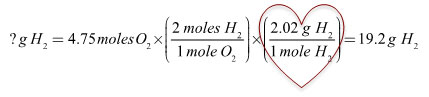
In this problem, no step in dimensional analysis was required for Phase 1 because you were given moles to start with. In Phase 3, you used molar mass to solve for your desired unit of grams.
