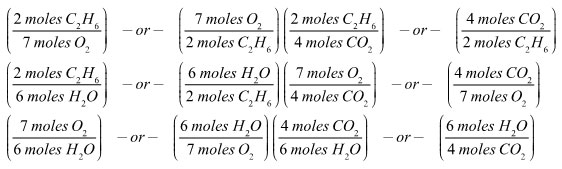A balanced chemical equation tells you the formulas for each chemical. Each formula is made up of one or more elements. The number of atoms of each element in a particle of that chemical is called a subscript.
For example, look at the formula for water. What are the subscripts used in the compound?

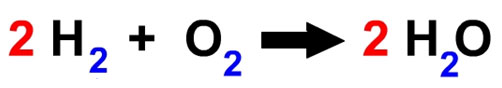
Now look at the entire equation for the formation of water. What coefficients are used?

Remember that the coefficients can tell the story of both particles and moles. For example, the following can be said:
![]() Look at the two graphs below. Drag the appropriate axis title for each x-axis onto the graph.
Look at the two graphs below. Drag the appropriate axis title for each x-axis onto the graph.
![]() Coefficients are all about mole ratios. Fill in the mole ratios below based on the balanced equation for the formation of water.
Coefficients are all about mole ratios. Fill in the mole ratios below based on the balanced equation for the formation of water.
Notice that you can also represent this ratio as follows:
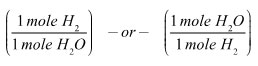
For the sake of consistency, you will always use the coefficients exactly as they are in the balanced equation even when they can be reduced. Using the ratio will not affect any math you do, but it will help you keep track of where your numbers came from.
How many mole ratios can you come up with for the balanced equation representing the combustion of ethane?
2 C2H6 + 7 O2→ 4 CO2 + 6 H2O
Interactive popup. Assistance may be required.
There are six possible mole ratios. Each ratio can be presented one of two ways.