When writing chemical equations, it is important that the number of atoms on each side of the reaction balance using the lowest whole number coefficients. Here is one method for establishing the correct coefficients in your equation.
Remember that when balancing a chemical equation, you can’t add new elements or change the ones already given to you. The only numbers you can change are the coefficients for each chemical, which tell you how many of each substance you need for the reaction to be balanced. In the equation for the formation of water, the coefficients below are 2, 1, and 2.
2 H2 + 1 O2 → 2 H2O
![]() Click on the next button to begin the interactive.
Click on the next button to begin the interactive.
Try some equations on your own.
Interactive popup. Assistance may be required.
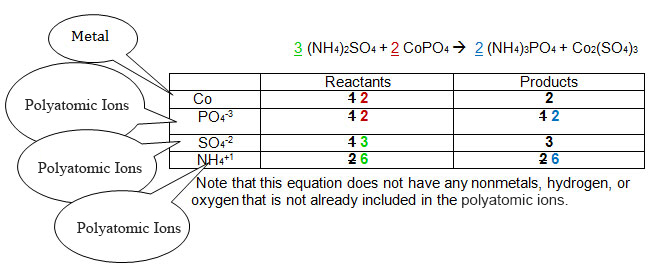
The original numbers are in black. The sequence of steps required to balance the equation appear in the order red, then blue, then green. See if you can keep track of how the equation was balanced.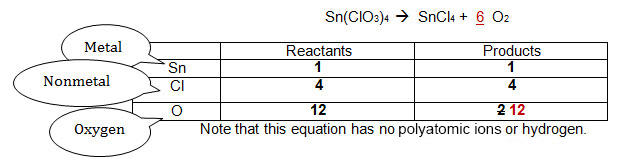

Interactive popup. Assistance may be required.
The original numbers are in black. The sequence of steps required to balance the equation appear in the order red, then blue, then green. See if you can keep track of how the equation was balanced.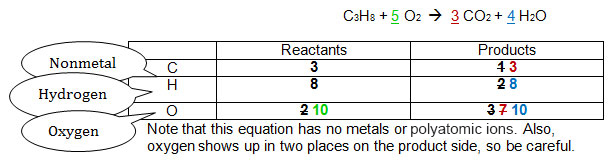

Interactive popup. Assistance may be required.
The original numbers are in black. The sequence of steps required to balance the equation appear in the order red, then blue, then green. See if you can keep track of how the equation was balanced.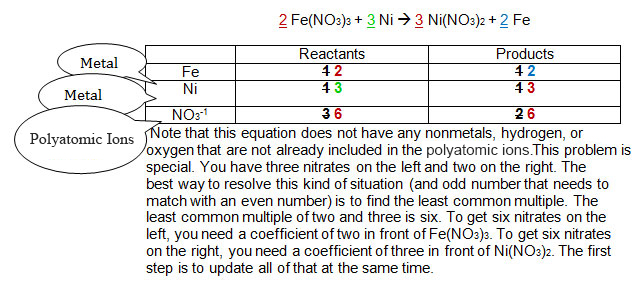

Interactive popup. Assistance may be required.
The original numbers are in black. The sequence of steps required to balance the equation appear in the order red, then blue, then green. See if you can keep track of how the equation was balanced.