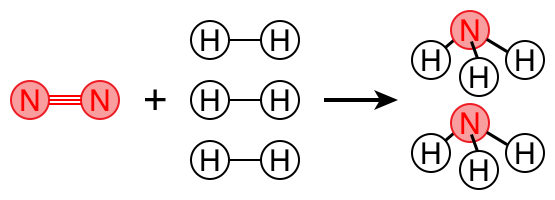
Below is a balanced equation. Try writing the balanced chemical equation for the reaction of nitrogen and hydrogen to produce ammonia.


Consider the reaction for the combustion of methane, CH4. Try to determine the coefficients that will make it a balanced equation before clicking through the two links below it.
___ CH4 + ___ O2 → ___ CO2 + ___ H2O
The following link will give you more practice on balancing equations.