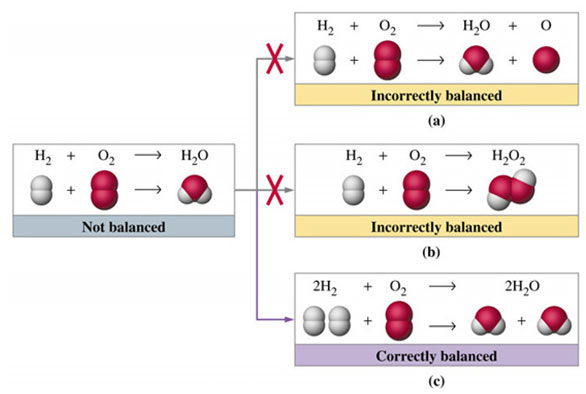
Source: Examples and non-examples of ways to balance a reaction, Prentice Hall
Review the image below, depicting representations for balancing the formation of water reaction.

Source: Examples and non-examples of ways to balance a reaction, Prentice Hall
In summary, you cannot balance a reaction by adding or changing the elements involved. The only thing you can do is change the number of particles, or moles of particles, participating so that mass is conserved. Look at the image below displaying a chemical equation with coefficients and subscripts.
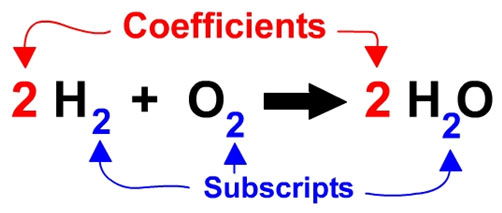
Source: Numbers in Balanced Reactions, balancing equations info
Remember, do not adjust subscripts when balancing equations.
![]() Now, watch the video on the law of conservation of mass.
Now, watch the video on the law of conservation of mass.
Source: The Law of conservation of mass, Power of 10Texas, YouTube
Click on the link to access the simulation. Click through the "Interactive Simulation for Balancing the Formation of Water."
![]() Confirm that mass is conserved in the equation for the formation of water by filling in the table below. Click on the blank cells in the table to check your work.
Confirm that mass is conserved in the equation for the formation of water by filling in the table below. Click on the blank cells in the table to check your work.