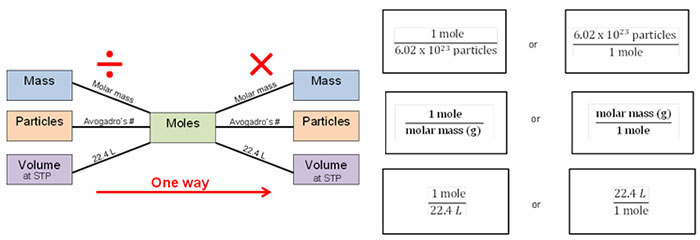
Complete the following practice problems. Remember the mole bridge, the conversions factors, and the steps for dimensional analysis. Use your Chemistry STAAR reference material to help you.

Dimensionsal Analysis Steps
Interactive popup. Assistance may be required.
grams → moles → particles (atoms)
Interactive popup. Assistance may be required.
25.0 g Au over 1 25.0 g Au 1 ×1 mole Au over 197.0 g Au 1 mole Au 197.0 g Au ×6.02 × 1023 atoms Au over 1 mole Au 6.02 × 1023 atoms Au 1 mole Au = 7.64 × 1022 Au atoms
Interactive popup. Assistance may be required.
particles (atoms) → moles → grams
Interactive popup. Assistance may be required.
7.56 × 1023 atoms Li over 1 7.56 × 1023 atoms Li 1 × 1 mole Li over 6.02 × 1023 atoms Li 1 mole Li 6.02 × 1023 atoms Li ×7.0 g Li over 1 mole Li 70.0 g Li 1 mole Li = 8.79 g Li
Interactive popup. Assistance may be required.
particles (molecules) → moles → grams
Interactive popup. Assistance may be required.
7.80 × 1045 Molecules H2O over 1 7.80 × 1045 Molecules H2O 1 × 1 mole H2O over 6.02 × 40.1 g Ca 1 mole H2O 6.02 × 1023 Molecules H2O ×18.0 g H2O over 1 mole H2O 18.0 g H2O 1 mole H2O = 2.33 × 1023 g H2O
Interactive popup. Assistance may be required.
grams → moles → particles (atoms)
Interactive popup. Assistance may be required.
13.3 g Ca over 1 13.3 g Ca 1 × 1 mole Ca over 6.02 × 40.1 g Ca 1 mole Ca 40.1 g Ca ×6.02 × 1023 atoms Ca over 1 mole Ca 6.02 × 1023 atoms Ca 1 mole Ca = 1.99 × 1023 Ca atoms
Interactive popup. Assistance may be required.
grams → moles → liters
Interactive popup. Assistance may be required.
2.3 g NO2 over 1 2.3 g NO2 1 × 1 mole NO2 over 46.0 g NO2 1 mole NO2 46.0 g NO2 ×22.4 L NO2 over 1 mole NO2 22.4 L NO2 1 mole NO2 = 1.1 L NO2
Interactive popup. Assistance may be required.
liters → moles → grams
Interactive popup. Assistance may be required.
13.3 g Ca over 1 46.8 L N2O5 1 × 1 mole Ca over 6.02 × 40.1 g Ca 1 mole N2O5 22.4 L N2O5 ×6.02 × 1023 atoms Ca over 1 mole Ca 108.0 g N2O5 1 mole N2O5 = 226.0 g N2O5
Interactive popup. Assistance may be required.
liters → moles → particles (molecules)
Interactive popup. Assistance may be required.
52.0 L H2 over 1 52.0 L H2 1 × 1 mole H2 over 22.4 L H2 1 mole H2 22.4 L H2 ×6.02 × 1023 Molecules H2 over 1 mole H2 6.02 × 1023 Molecules H2 1 mole H2 = 1.4 × 1024 molecules H2
![]() Use the information given and your periodic table to complete the table. Be sure to use the correct number of significant figures.
Use the information given and your periodic table to complete the table. Be sure to use the correct number of significant figures.