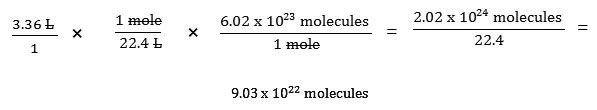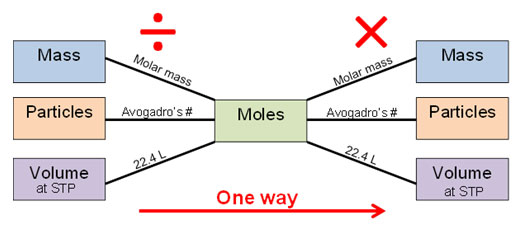
In all the previous examples, you only had to use one conversion factor. That is not always the case! For example, you may be given the volume of a gas at STP and be asked to find the number of molecules. The mole is essential in solving this type of problem. You have seen an image like the one below before. It is often referred as a “mole bridge.”

Notice the red arrow and the words “one way.” When you use the mole bridge, you start out with what you are given on the left, and then divide by the conversion factor found on the black line; this will give you moles. Then, find the desired unit on the right and multiply by the conversion factor on the line that leads to the desired unit.
For example, if you were given the volume of a gas at STP and asked to find the number of molecules, the path on the mole bridge would be as follows:
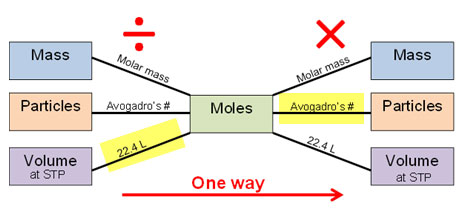
In other words, you would divide your given volume by 22.4 L and then multiply your answer by Avogadro’s number.
If you use dimensional analysis and you make sure your units are canceling, you will be able to easily solve any problem you are given. Just remember, converting to moles has to be the middle step.
Using dimensional analysis, you would set up the example from above as follows:
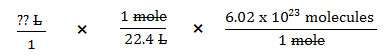
Now, let’s look at a real problem.
How many oxygen molecules are in 3.36 L of oxygen gas at STP?