Source: STAAR Reference Material, Texas Education Agency
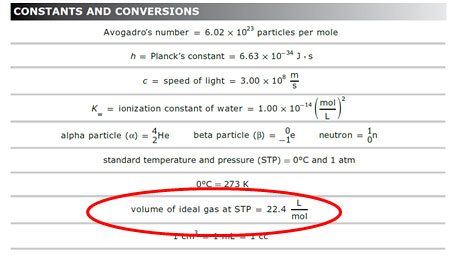
You may be asked to determine the volume, in liters, of a certain gas at STP (standard temperature and pressure.) When converting from liters to moles or moles to liters, you will use the equality 1 mole=22.4 L for a gas at STP. This number can be found on the Constants and Conversions section of the STAAR reference material.

Source: STAAR Reference Material, Texas Education Agency
This equality can be written as a set of two conversion factors. They are as follows:
1 mole over 22.4 L 1 mole 22.4 L or 22.4 L over 1 mole 22.4 L 1 mole
Remember these conversion factors can only be used at standard temperature and pressure.
Let’s practice a few mole-molar conversions. You will use the steps for dimensional analysis to help solve these problems.
What is the volume, in liters, occupied by 0.030 moles of a gas at STP ?
How many moles of argon are present in 11.2 L of argon gas at STP?