Source: Oxygen, Water Molecule, Sodium Chloride, Wikimedia Commons
The mole is
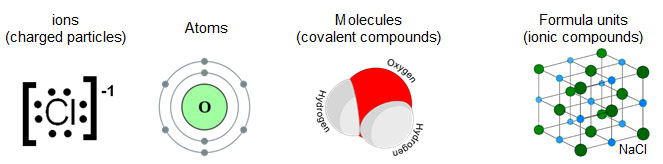
These things can be items such as

Source: Oxygen, Water Molecule, Sodium Chloride, Wikimedia Commons
When converting between particles and moles, you will use the equality 1 mole = 6.02 x 1023 particles. This number is given in the section titled Constants and Conversions on the Chemistry STAAR reference material.

Source: STAAR Reference Material, Texas Education Agency
This equality can be written as a set of two conversion factors. They are as follows:
1 mole over 6.02 × 1023 particles 1 mole 6.02 × 1023 particles or 6.02 × 1023 particles over 1 mole 6.02 × 1023 particles 1 mole
Remember that the words ions, atoms, molecules, or formula units can be substituted in place of the word particles.
Let’s practice a few mole-particle conversions. You will use the steps for dimensional analysis to help solve these problems.
How many moles of magnesium are in 3.01 x 1022 atoms of magnesium?
How many molecules are there in 4.00 moles of glucose, C6H12O6?
Now You Try!
How many molecules are found in 3.2 moles of CH4?