
When a substance undergoes alpha radiation it gives off alpha particles. An alpha particle is made up of 2 protons and 2 neutrons. This means that an alpha particle has a mass of 4 and a charge of +2. An alpha particle can be written three ways:
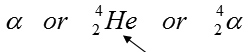
We use the symbol for helium because an alpha particle is the same as a helium nucleus.
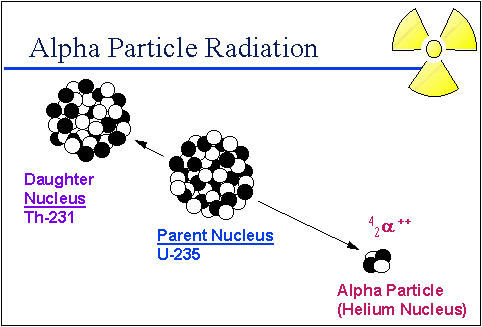
Source: Alpha particle radiation, University of Michigan Student Chapter of the Health Physics Society
The diagram above shows the original nucleus (parent) of unstable uranium-235. U235 naturally gives off two neutrons and two protons which is the same as a helium nucleus which has a positive charge. By losing the two protons uranium, which has 92 protons in the nucleus, it changes into thorium–231, which has 90 protons in the nucleus.
Other elements which are alpha emitters are:
Americium-241, used in smoke detectors
Californium-252, used to inspect luggage for explosives
Radium-226, used to make lightning rods more effective
Thorium-220, used for coloring in glassware
Uranium-235, used in nuclear power plants
Alpha particles are relatively heavy and large. So as they travel through air, the distance they can travel is just a few centimeters. Because of their size alpha particles are easily stopped by a single sheet of paper as demonstrated in the graphic below.
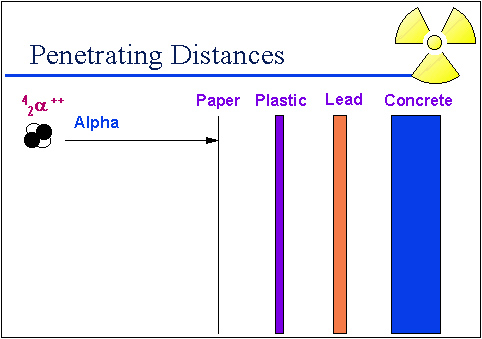
Source: Penetrating distances, University of Michigan Student Chapter of the Health Physics Society
Alpha particles cannot penetrate the outer layer of the skin. So they do not present a hazard from exposure from outside the body. However, if ingested or inhaled they can become very dangerous by penetrating soft tissues inside the body.

Now answer the following questions.