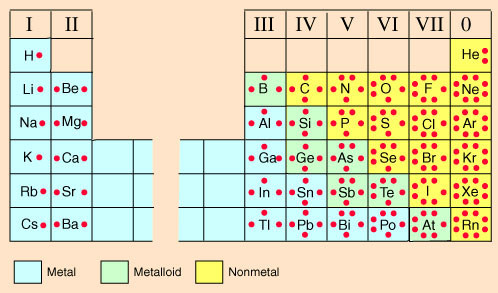
Lewis valence dot diagrams, also called Lewis dot diagrams, are models that show only the valence electrons. There are often more electrons in the lower energy levels (energy levels that are closer to the nucleus) that are not involved with bonding and, therefore, not shown on an electron dot diagram.
Look at the periodic table on the left and compare it to the periodic table from the STAAR Reference Material on the right.

Notice how the group numbers on the STAAR reference chart, 1A, 2A, 3A, etc., match the number of valence electrons as shown on the table with the electron dot diagrams. (The exception is helium, He, which only has one energy level or orbital. Hence, helium has only two valence electrons, but still a full valence shell.) The element's symbol is in the center of the dot diagram. The dots around the symbol represent the valence electrons. Notice how the dots are not in a circle around the symbol, but more of a box with up to two electrons on each side and, at the most, eight total valence electrons.
![]() Watch the following video to learn how to draw the Lewis valence electron dot structure for oxygen. Pay close attention to the order in which the dots are placed around the symbol.
Watch the following video to learn how to draw the Lewis valence electron dot structure for oxygen. Pay close attention to the order in which the dots are placed around the symbol.
Did you notice in the video that one dot was placed on each of the four sides until they were all filled? This was intentional. Remember, electrons are all negatively charged and like charges repel each other. Electrons will fill orbitals one at a time until there is a lack of open space to minimize these repellent negative charges. When drawing the dots, follow the order as shown in the diagram below. Note, the X represents any element symbol.
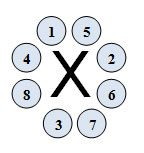
Depending on the source, the dots may be placed in a slightly different arrangement. Do not worry too much about the placement; just make sure you get the correct number of dots drawn.
In the following activity, practice building Lewis valence electron dot structures. When the element name appears, click on the corresponding chemical symbol. Then, select the correct number of valence electrons (dots) for that element. Use the periodic table in the STAAR Reference Material to determine the number of valence electrons.
![]() Click on "next step" to begin the interactive.
Click on "next step" to begin the interactive.