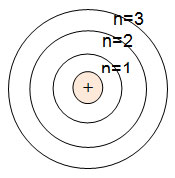
Bohr found that the closer an electron was to the nucleus, the less energy it had. Bohr assigned numbers to the energy levels. The higher the energy-level number, the father away from the nucleus it is. The diagram below shows the energy levels of a Bohr model.

Bohr also hypothesized that the various energy levels could hold a certain amount of electrons. Use the following interactive to explore how many electrons the first three energy levels can hold.
![]() Click on an electron, and drag it to the energy levels. Continue this process until you have placed all the electrons in the atom. Once you have finished, answer the questions below.
Click on an electron, and drag it to the energy levels. Continue this process until you have placed all the electrons in the atom. Once you have finished, answer the questions below.
How many electrons can the first energy level (one closest to the nucleus) hold?
Interactive popup. Assistance may be required. 2
How many electrons can the second energy level hold?
Interactive popup. Assistance may be required. 8
How many electrons can the third energy level hold?
Interactive popup. Assistance may be required. 18
Can you add electrons to the second energy level without the first being full?
Interactive popup. Assistance may be required. No
Can you add electrons to the third energy level without the first and second being full?
Interactive popup. Assistance may be required. No
After completing the interactive, you can see that the first energy level can hold two electrons. Eight electrons fill level two, and 18 electrons fill level three. In general, each energy level must be full before electrons are placed on the next level. (You will learn more about electron configuration in other lessons in this course.)
Placement of the electrons can get complicated with elements of more than 18 electrons. For the purpose of this lesson, the discussion and examples will be limited to elements with atomic numbers of 1 to 18.
Now, let’s look at an example using sulfur. To know how many electrons are in which energy level, you must first know how many electrons you have. To find out, view the periodic table and look up the atomic number.
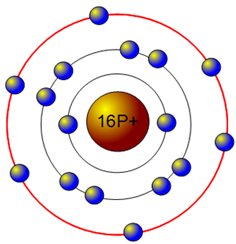
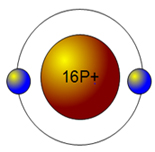
Sulfur has the atomic number 16; therefore, it has 16 protons and 16 electrons. The first energy level will fill up with two electrons.
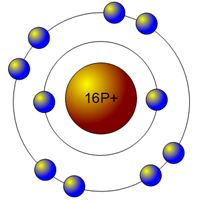
The second energy level fills up with eight electrons.
That takes care of 10 out of the 16 electrons. Now, since there are only six electrons left and the 3rd energy level has room for 18 electrons, the remaining six electrons will go in the 3rd energy level in the atom's Bohr model.