

Niels Bohr knew that Rutherford’s model of the atom was not accurate. Bohr used Max Planck and Albert Einstein’s findings along with his understanding of emission and absorption spectra of chemical elements to develop his model of the atom.
Bohr assumed that Rutherford’s ideas of the electrons orbiting the nucleus were correct. He proposed that electrons existed in distinct energy levels, or shells, around the nucleus and that each shell has a different amount of energy. Bohr also concluded that the energy of an electron is quantized, meaning an electron can be in one energy level or another but not in between energy levels.
Bohr hypothesized that when an electron is in the shell, it does not give off nor absorb energy. This is called the electron’s ground state. He went on to propose that an electron could move to a higher energy level, or shell, by absorbing energy. This is called the electron’s excited state. The electron can return to its ground state by releasing the energy that it absorbed. The light emission process happens when the electron releases energy.
The image below shows the ground and excited states in the Bohr model.
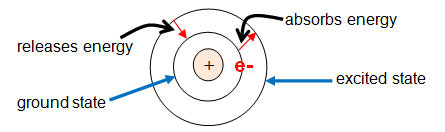
Bohr said that sometimes the energy released by an electron occupies part of the electromagnetic spectrum that is detected as visible light. This explains the emission and absorption spectrum.
Let’s look at the emission spectra of hydrogen again.

Hydrogen only has one electron, yet in the emission spectra there are five lines. Why is this? Electrons can "jump" more than one energy level. The variations in the amount of energy that is absorbed and then released by the electron when it jumps to different levels are seen as different colors.
Let’s summarize Bohr’s conclusions.
Bohr’s model explained the absorption and emission spectrum of hydrogen and ions that contain only one electron such as He+, Li2+, and Be3+ ions, but his model did not fit the spectra produced by atoms with more than one electron. Bohr’s model also could not explain why the electrons in the ground state did not emit electromagnetic energy. Bohr’s idea that electrons travel in discrete energy levels was later proven to be incorrect.
Although Bohr’s model was proven to be incorrect, it is still used today as an introduction to the structure of the atom because it provides a simplistic model of the atom and the behavior of electrons.
Sources for images used in this section, as they appear, from top to bottom: