The physicist Niels Bohr built on the work of other scientists, including Rutherford, Planck, and Einstein.

Have you ever used a prism to separate light? Visible light contains light of many different frequencies. When light is passed through a prism, the different frequencies are separated into a spectrum, or range of wavelengths. The type of spectrum depends on the source of light. When elements are in a gas phase and are excited by heat or electricity, they emit light at various frequencies. The different frequencies show up as individual lines on an emission spectrum.
The image below shows a basic diagram of how emission spectra are obtained.
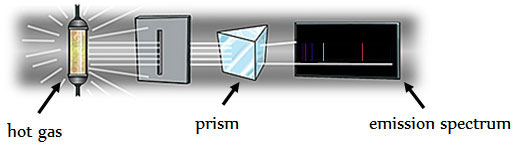
The emission spectrum is different for each element. The emission spectrum is like a fingerprint for the element.
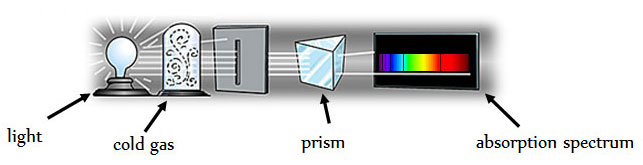
Each element also has unique absorption spectra. An absorption spectrum is obtained by passing light through a cold element and then through a prism. The image below shows a basic diagram of how absorption spectra are obtained.
The diagrams below show the emission spectrum and absorption spectrum for hydrogen. Notice that the images are opposite of each other; the dark areas in the emission spectrum are colored and vice versa in the absorption spectrum.

Sources for images used in this section, as they appear, from top to bottom: