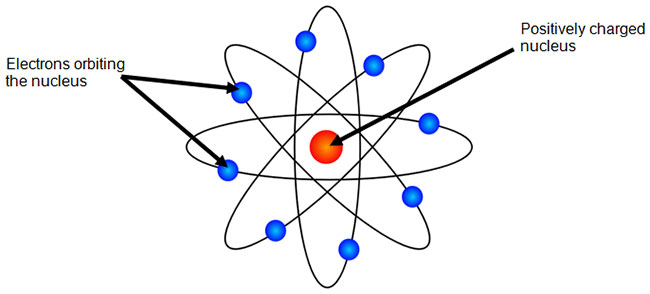
In 1907, Ernest Rutherford’s experiments disproved J.J. Thomson’s Plum Pudding model of the atom. Rutherford concluded the following:
The image below shows Rutherford’s model of the atom.

Even though Rutherford’s model was an improvement to Thomson’s model, there were a few problems with his findings. According to laws of physics, electrons moving around the nucleus should release electromagnetic energy. If an electron were releasing or losing energy, it would slowly spiral into the positively charged nucleus as the image below shows.
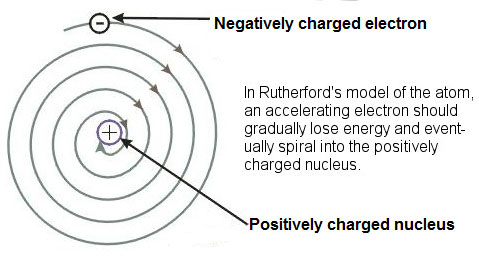
If electrons spiraled into the nucleus, all matter in the universe would fall apart.
In the early 1900s, a German physicist named Max Planck was studying the electromagnetic spectrum. Planck’s theory summarized that energy was not continuous and flowing but instead contained in tiny energy units, which he called quanta. He developed a constant, which is now known as Planck’s constant, to relate the energy of quanta to the frequency of light. (You will learn more about Planck and his theories in a later lesson.)
Albert Einstein used Planck’s theory to determine the photoelectric effect. The photoelectric effect states the following:
Planck’s and Einstein’s theories were supported by the emission and absorption of light given off by different elements.
Sources for images used in this section, as they appear, top to bottom: