
Electromagnetic radiation not only takes the form of waves, it also takes on the properties of particles. Particles of radiation are called photons . The energy of a photon depends on its electromagnetic energy; it is directly proportional to the photon's frequency.
If you add enough heat to an element, electrons move between energy levels within an atom. The difference in the energy between the lower energy atom and the higher energy atom is emitted or absorbed in the form of photons.

![]() To see an animation of the electron in Hydrogen jumping energy levels, go to
To see an animation of the electron in Hydrogen jumping energy levels, go to

and click on Excite 1-2, 1-3 or 1-4.
Because the energy levels have definite energies, the photons also have definite energies. These energies result in different colors, which are characteristic of the type of atom involved.
Sodium vapor lights are used for street lights in many cities and towns. When heat is added to the sodium vapor in the lamp, the electrons jump to higher energy levels and emit photons with an orange-yellow tint. Sodium vapor lamps use less energy than the older blue colored mercury vapor lamps.

Source: Sodium vapor light, Tilman Kluge,
Wikimedia Commons

Source: Mercury vapor light, Torindkflt
So why does the sodium vapor light use less energy than the mercury vapor light? Let’s look at the electromagnetic spectrum again. Remember: Energy is directly proportional to frequency. So as the frequencies of the waves increase, the energy of the photons increase.
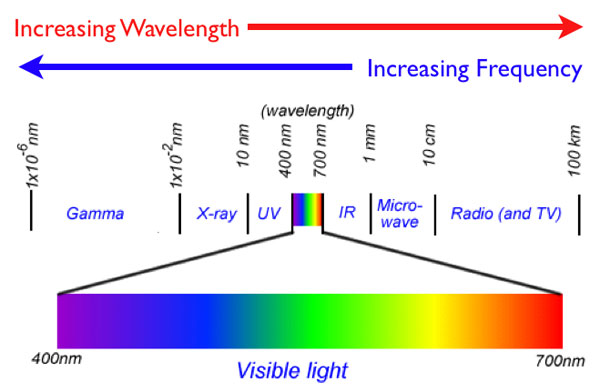
Source: Modified from: Electromagnetic Spectrum, Edbuzz
In the image above, which color light is emitting at a higher energy, yellow, or blue?
Interactive popup. Assistance may be required.
BLUE
So a blue mercury vapor lamp will emit at higher energies than a yellow sodium vapor lamp.
What happens to the energy of the photons as frequency increases?
Interactive popup. Assistance may be required.
As frequency increases, energy increases.