![]() How do you know where an electron is most likely to be found? Like most questions in chemistry, you can turn to the periodic table for answers.
How do you know where an electron is most likely to be found? Like most questions in chemistry, you can turn to the periodic table for answers.
First, you need to know the energy level number. Do you remember what on the periodic tables identifies the number of energy levels?
There are seven periods or rows on the periodic table, so there are seven possible energy levels.
Secondly, you need to know how to identify the sublevels. Again, you can turn to the periodic table for answers. The sublevels are not readily identified on the periodic table, but they are relatively easy to learn. The periodic table can be divided into different sections, or “blocks.” Each block represents a different sublevel. The image below shows a color-coded periodic table identifying the different sublevels.
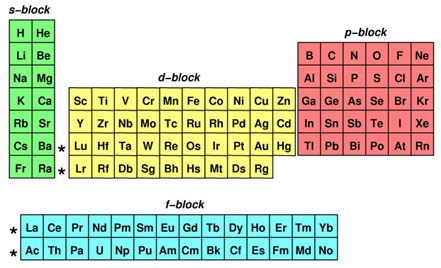
This information is not on the periodic table, but it is easy to memorize. Notice on the periodic table above, Helium has been moved over to the s block. Helium, a noble gas, is normally located in group 18, but it does not have a p sublevel, so it is in the s block.
Remember that the period number identifies the energy level number. For sublevel d, the energy level number is 1 minus the period number. For sublevel f, the energy level number is 2 minus the energy level number. Look at the periodic table below.
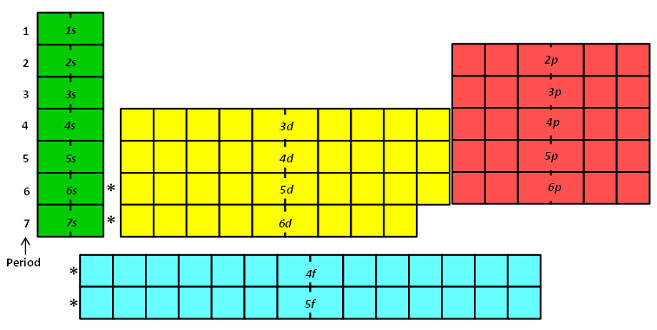
The number of orbitals for each sublevel is a set amount. Remember that in section two you learned that the each sublevel has the following number of orbitals.
Sublevel |
Orbitals |
s |
1 |
p |
3 |
d |
5 |
f |
7 |
In section two of this lesson, you learned that each orbital can have no more than two electrons. Therefore, each sublevel has a set number of electrons. The chart below shows the number of electrons per sublevel.
Sublevel |
Number of Orbitals |
Electrons per Sublevel |
s |
1 |
2 |
p |
3 |
6 |
d |
5 |
10 |
f |
7 |
14 |
The periodic table can serve as a reminder of the number of electrons per sublevel. Look at the color-coded periodic table again.

How many columns are in the s block?

How many columns are in the p block?

How many columns are in the d block?

How many columns are in the f block?

Sources of images used for this section as they appear, top to bottom: