
How do you keep track of the location of the electrons? The way the electrons are arranged around the nucleus of an atom is called the electron configuration. As you recall, the protons and neutrons are found inside the nucleus of an atom, while the electrons are constantly moving around the nucleus. The nucleus and the electrons interact to form the most stable arrangement possible, the ground state, which requires the least amount of energy.
There are four main concepts that you must remember in electron configuration. They are energy levels, sublevel, orbitals, and number of electrons per sublevel. To help you understand electron configuration, we will use an analogy of an apartment building.

The energy levels, or shells, are essentially the same as Bohr’s energy-level numbers. The letter n and a whole number are used to represent the energy levels. For example, n = 1 represents the first energy level. This number describes the average distance from the nucleus. The larger the value of n, the further away the energy level is from the nucleus and the more energy the level contains. In our analogy, the energy levels would be the floors in the apartment building.
Each energy level, or shell, is divided into sublevels. The terms sublevel and subshell are used interchangeably. The sublevels are represented by the letters s, p, d, and f. Each energy level has certain sublevels. The chart below shows the sublevels that make up the first four energy levels.
| Energy Level | Sublevels |
| n = 1 | s |
| n = 2 | s and p |
| n = 3 | s, p, and d |
| n = 4 | s, p, d, and f |
Energy levels that are higher than four would contain additional sublevels such as g and h. We will not worry about these sublevels at this time because no known atom, in its ground state, would have electrons that occupy those sublevels.
In our analogy, the sublevels would be the apartments located on each floor of the building.
Each sublevel is made up of orbitals. Each sublevel has a different number of orbitals. The chart below shows the number of orbitals for each sublevel.
| Sublevels | Orbitals |
| s | 1 |
| p | 3 |
| d | 5 |
| f | 7 |
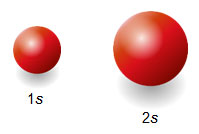
When scientists describe orbitals in an atom, they use the energy level number and the sublevel letter. For example, 1s denotes the first energy level and sublevel s, while 3p would denote the third energy level and the sublevel p. Orbitals are classified by their shape. For example, s orbitals are spherical in shape. The difference between 1s, 2s, etc. is their size as shown in the figure below.
Orbitals found in the p subshell are dumbbell, or figure-eight, shaped. Remember, in each p subshell there are three orbitals, so there are three dumbbell shapes. Atoms are three dimensional objects, and the orbitals are oriented around the different axes. The image below shows the three p orbitals and the complete p sublevel containing all three orbitals.
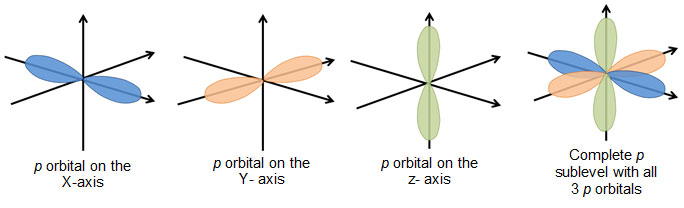
The d and f orbitals have more complex shapes than the s and p orbitals, so those will not be shown in this lesson. The main idea to remember is that each sublevel has a certain number of orbitals, and each orbital has a distinct shape.
In our apartment analogy, the orbitals would be the individual rooms in each apartment.
Each orbital can hold no more than two electrons. So, each s sublevel can have two electrons, each p sublevel can hold six electrons, etc. The chart below shows the number of electrons per orbital and per sublevel.
Sublevel |
Number of Orbitals |
Electrons per Sublevel |
s |
1 |
2 |
p |
3 |
6 |
d |
5 |
10 |
f |
7 |
14 |

In our apartment analogy, the number of electrons per orbital is the number of roommates in each room. The number of electrons per sublevel would be the total number of people living in the apartment.
The chart below summarizes the orbitals and electron capacity for the first four energy levels.
| Energy Level | Sublevels | Number of Orbitals | Number of Electrons Per Sublevel | Maximum Number of Electrons per Energy Level |
1 |
s |
1 |
2 |
2 |
2 |
s |
1 |
2 |
8 |
p |
3 |
6 |
||
3 |
s |
1 |
2 |
18 |
p |
3 |
6 |
||
d |
5 |
10 |
||
4 |
s |
1 |
2 |
32 |
p |
3 |
6 |
||
d |
5 |
10 |
||
f |
7 |
14 |
Sources of images used for this section as they appear, top to bottom: