Niels Bohr’s atomic model had limitations. His model was unable to explain the spectra produced by atoms with more than one electron. His model also could not explain why electrons in the ground state did not give off electromagnetic energy. A more complex model based on mathematics and physics was developed. The current model of the atom is called the quantum mechanical model. It is also often referred to as the electron cloud model.
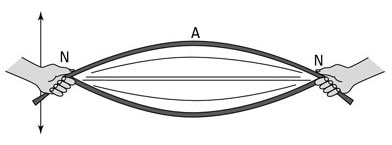
In 1927, scientists started studying the wave-like characteristics of electrons. Electrons behave like standing waves. Standing waves are waves that have two fixed points and, therefore, oscillate in place. An everyday example of a standing wave is a rope held by both ends. If one person flicks his or her end of the rope, a wave will form that looks similar to the image below.
The part of the wave near the fixed points does not appear to move much. This is called the node and is represented by the letter N in the picture. The maximum displacement of the wave is called the antinode and is represented by the letter A in the picture.

The more energy put into the wave, the more nodes and antinodes there are. If the rope was flicked with a lot of energy, it would look like the image below.
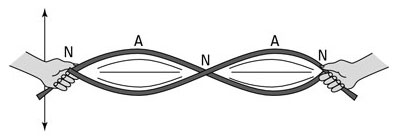
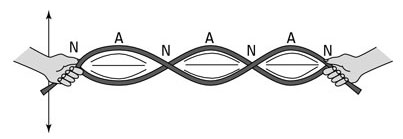
Finally, if the rope was flicked with even more energy, it would have many nodes and antinodes like the image below.
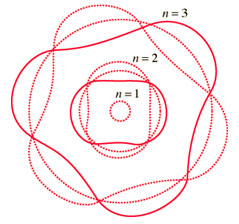
If you apply this standing wave-like behavior of electrons to the Bohr model, it might look something like the image below. The letter n stands for the energy level; in this image, the first three energy levels are shown.
Electrons are extremely small and move extremely fast, so there is no way of knowing exactly where an electron is at any exact moment. However, by using math and physics, scientists were able to determine the probability, or best guess, as to the location of an atom’s electrons. Bohr’s orbits, or energy levels, were replaced with orbitals. An orbital is a volume of space in which the electron is likely located. Picture electrons moving fast in many directions around the nucleus. The electrons become a blur of motion. You might picture something like the image below.
Notice that the blur of motion made by the electrons looks like a cloud; this is why it is referred to as the electron cloud.
Sources of images used for this section as they appear, top to bottom: