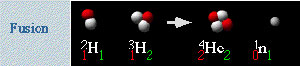
Source: Fusion, Lawrence Berkeley National Laboratory
Nuclear fission and fusion can be expressed in equations. The following graphic is a diagram for the deuterium-tritium fusion reaction paired with the symbols for each of the substances in the reaction.

Source: Fusion, Lawrence Berkeley National Laboratory
In this case deuterium with one proton (red) and one neutron (white) is represented by the symbol: The symbol for hydrogen preceded by a superscript 2 and a subscript 1. It is followed by a subscript 1. 2 1 H1
The number 2 represents the atomic mass. The lower left 1 represents the number of protons, while the lower right 1 represents the number of neutrons.
Likewise the symbol for tritium is:
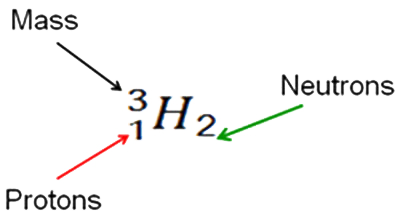
The next symbol is for helium: The symbol for helium preceded by a superscript 4 and a subscript 2. It is followed by a subscript 2. 4 2 He2
How many neutrons are in this isotope of helium? Click for answer: Interactive button. Assistance may be required. __________ 2
How many protons are in this isotope of helium? Click for answer: Interactive button. Assistance may be required. __________ 2
What is the mass number for this isotope of helium? Click for answer: Interactive button. Assistance may be required. __________ 4
The final symbol represents a single neutron: The letter n preceded by a superscript 1 and a subscript 0. It is followed by a subscript 1 1 0 n1
If we put this altogether in an equation we have: Hydrogen 2 plus Hydrogen 3 yeilds Helium 4 plus one neutron plus energy 2 1 H1 + 3 1 H2 → 4 2 He2 + 1 0 n1 + energy
On the left side of the equation, we have a total of 3 neutrons. On the right side, this helium isotope has only two neutrons. We must show the emitted neutron in order to balance the equation.
How would you write the equation for the deuterium-deuterium reaction? Below is the basic equation. Drag and drop the correct numbers for deuterium and helium in the correct location in the equation. There are more number choices than you will need.
Remember, deuterium has one proton and one neutron.
![]()
We can also express nuclear fission in the form of an equation. The following graphic is a diagram showing a Uranium 235 nucleus being split into Xenon 134 and Strontium 100. There are two neutrons emitted as a result of the reaction.

Source: Fission, Lawrence Berkeley National Laboratory Original
![]() What do these numbers represent in the symbol for U 235?
What do these numbers represent in the symbol for U 235?
Here is the reaction in equation format:
Uranium 235 plus one neutron yields Xenon 134 plus Strontium 100 plus one neutron plus one neutron plus energy 235 92 U143 + 1 0 n1 → 134 54 Xe80 + 100 38 Sr62 + 1 0 n1 + 1 0 n1 + energy
Notice that you have a total of 144 neutrons on the left side of equation. Adding the neutrons from Xe and Sr only gives us 142. Therefore we show the 2 isolated neutrons are emitted so that the equation is balanced.