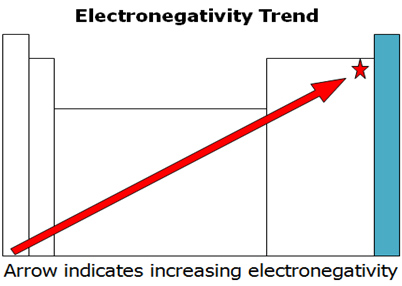
Electronegativity is the tendency of an atom to attract a bonding pair of electrons. Electronegativity is normally measured in terms of the Pauling scale. The element with the highest electronegativity is fluorine with a value of 4.0. Cesium and francium have the lowest electronegativity each with a value of 0.7.

![]() Watch this video about ionization energy and electron affinity.
Watch this video about ionization energy and electron affinity.
Source: Overview: Atomic Size, Ionization Energy & Electron Affinity, Papapodcasts, YouTube
Examine the electronegativity trend graph and answer the following question in your notes.
What are the trends that occur within the periods and the groups?
Interactive popup. Assistance may be required. In a group, electronegativity increases as atomic number decreases. In a period, electronegativity increases as atomic number increases.
![]() Place the following elements in the correct order from lowest ionization energy to highest ionization energy.
Place the following elements in the correct order from lowest ionization energy to highest ionization energy.
Refer the periodic table provided for you in the STAAR resources.
STAAR Chemistry Reference Materials PDF