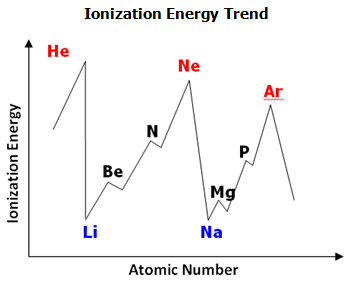
The ionization energy, sometimes called the ionization potential, is the energy required to completely remove an electron from an ion. If the electron is close to and tightly bound to the nucleus, then it will require more energy to remove than if the electron is farther away. So ionization energies are higher for atoms with tightly bound electrons.
The first ionization energy is the energy required to remove one electron from the atom. The second ionization energy is always greater than the first ionization energy. With regards to the periodic table, ionization energies increase from left to right across a period (decreasing atomic radius). Ionization energy decreases moving down a group (increasing atomic radius). The images below demonstrate these trends graphically.

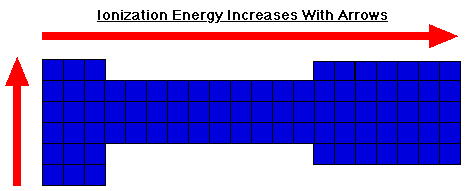
Source: Ionization energies, Chemviz, http://www.shodor.org/chemvis/ionization/students/background.html
![]() Watch this video about periodic trends.
Watch this video about periodic trends.
Source: Periodic Table Trend in Ionization Energy, kentchemistry, You Tube
Examine the ionization energy trend graph. Answer the following question in your notes.
What happens with ionization energies within periods?
Interactive popup. Assistance may be required. Within a period, as atomic number increases, ionization energies increase.