Source: Atomic radii, Dr. Walt Volland, http://www.800mainstreet.com/33/0003-008-00-elec-per.html
In chemistry, periodic trends are the tendencies of certain elemental characteristics to increase or decrease moving along a row or column of the periodic table of elements. The elemental characteristics discussed here are:
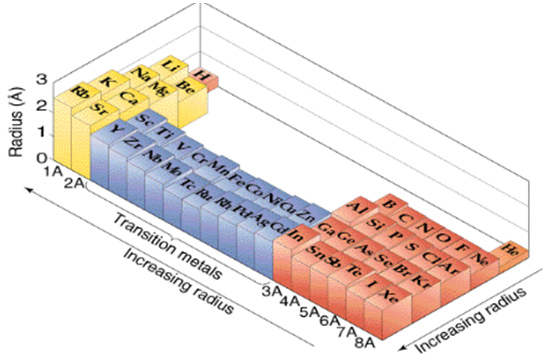
The atomic radius is the distance from the atomic nucleus to the outermost boundary of an adjacent atom. The atomic radius tends to decrease across a period. One reason it decreases is because the nuclear charge increases. This stronger nuclear charge attracts the orbiting electrons. Since the electrons are pulled in toward the center of the atom, the atomic radius is smaller.
The atomic radius usually increases while going down a group because of the addition of a new energy level. However, diagonally, the number of protons has a larger effect than the sizeable radius.
The image below graphically shows the trends of atomic radii across the periodic table.

Source: Atomic radii, Dr. Walt Volland, http://www.800mainstreet.com/33/0003-008-00-elec-per.html
Click on the link below for a dynamic visual demonstration of the trends of atomic radii.