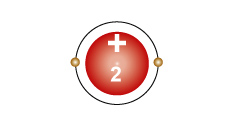
 Helium - He An atom with two protons in the nucleus, and two electrons in the first energy level. |
 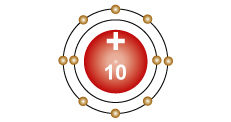 Neon - Ne An atom with ten protons in the nucleus, two electron in the first energy level, and eight electrons in the second energy level. |
 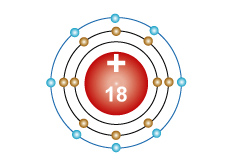 Argon - Ar An atom with ten protons in the nucleus, two electrons in the first energy level, eight electrons in the second energy level and eight electrons in the third energy level. |
 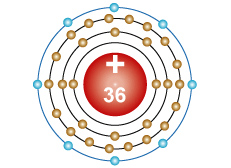 Krypton - Kr An atom with 36 protons in the nucleus, two electrons in the first energy level, eight electrons in the second energy level, eight electrons in the third energy level and eight electrons in the fourth energy level. |
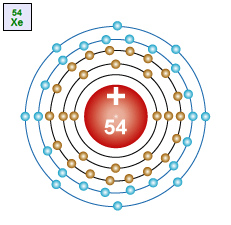 Xenon – Xe An atom with 54 protons in the nucleus, two electrons in the first energy level, eight electrons in the second energy level, eight electrons in the third energy level, eight electrons in the fourth energy level and eight electrons in the fifth energy level. |
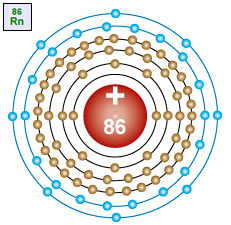 Radon – Rn An atom with 86 protons in the nucleus, two electrons in the first energy level, eight electrons in the second energy level, eight electrons in the third energy level, eight electrons in the fourth energy level, eight electrons in the fifth energy level and eight electrons in the sixth energy level. |
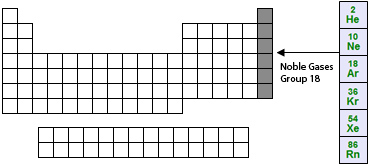
Location of the Noble Gases on the Periodic Table: