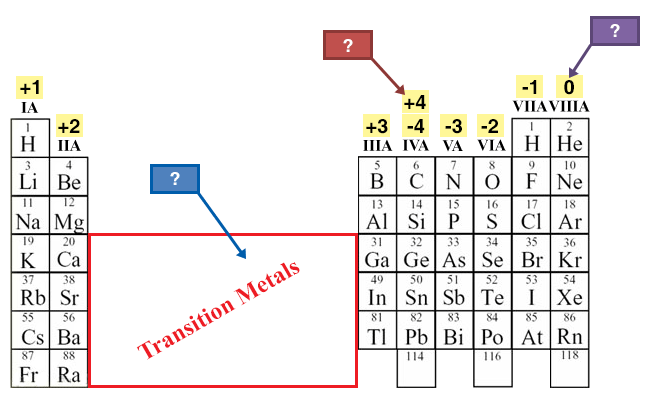

Transition Metals
These elements are transition metals. Transition metals are more complicated because these elements sometimes have different oxidation numbers. For example, iron can have a +2 or a +3 oxidation state.
4A (IVA)
The elements in group IVA have four valence electrons and can have an oxidation number of +4 or -4. Group 4 elements usually form covalent bonds, which you will learn more about in lesson 3 of this module.
8A (VIIIA)
Elements in group VIIIA are the noble gases and have a complete valence shell; therefore, they have an oxidation number of zero. These elements have very low reactivity.