Let’s start with the first column of elements. These are the alkali metals. Notice the 1A at the top of the column.
Let’s start with the first column of elements. These are the alkali metals. Notice the 1A at the top of the column.

If you look at images of the alkali metals, you will see that many of the elements have a similar appearance.

Source: Alkali Metals, Theodore W. Gray, Periodic Table.com
Elements in the same family also have similar properties that can be seen. Listed below are some characteristics that the alkali metals share.
Alkali metals have the following characteristics:
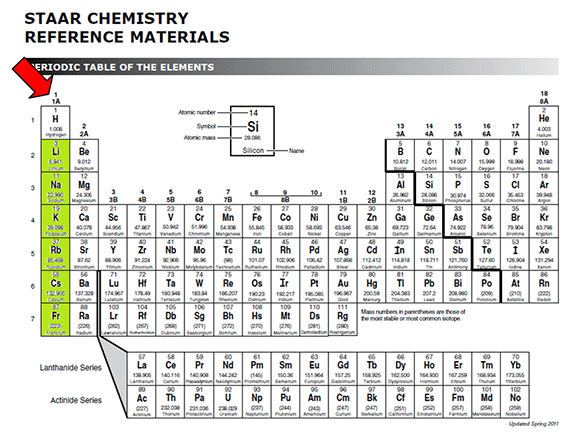
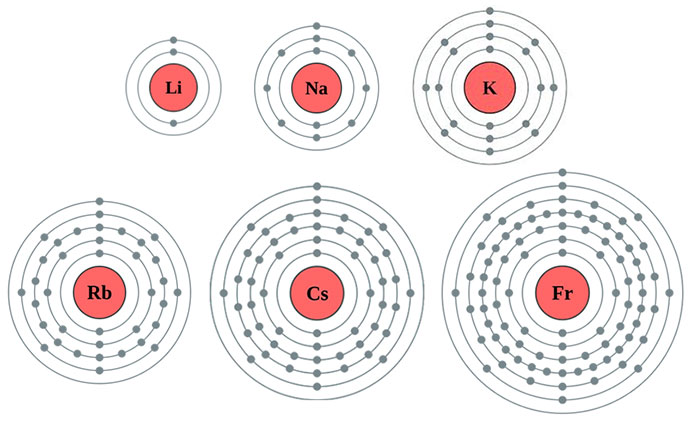
Look at the Bohr models of each of the alkali metals.
Source: All images from Wikimedia commons.
Notice that each element has one electron in the outer energy level.
Source: All images from Wikimedia commons.
Now, look at the electron configuration for each of the alkali metals.
Element | Electron Configuration |
Lithium | 1s22s1 |
Sodium | 1s22s22p63s1 |
Potassium | 1s22s22p63s23p64s1 |
Rubidium | 1s22s22p63s23p63d104s24p65s1 |
Cesium | 1s22s22p63s23p63d104s24p64d105s25p66s1 |
Francium | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p67s1 |
Notice that the outer most energy level of each element consists of an s sublevel with one electron.
Element | Electron Configuration |
Lithium | 1s22s1 |
Sodium | 1s22s22p63s1 |
Potassium | 1s22s22p63s23p64s1 |
Rubidium | 1s22s22p63s23p63d104s24p65s1 |
Cesium | 1s22s22p63s23p63d104s24p64d105s25p66s1 |
Francium | 1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p67s1 |
Now, let’s analyze the elements in the oxygen family. Notice the 6A at the top of the column.
If you look at images of the oxygen family, you will see that some of the elements have similar appearances.

Source: Alkali Metals, Theodore W. Gray, Periodic Table.com
You can see that the elements in the oxygen family have many similar characteristics.
Elements in the oxygen family
Look at the Bohr models of each element in the oxygen family.
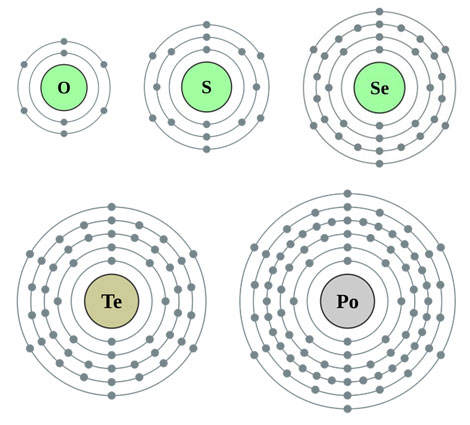
Source: Alkali Metals, Theodore W. Gray, Periodic Table.com
Notice that each element has six electrons in the outer energy level.
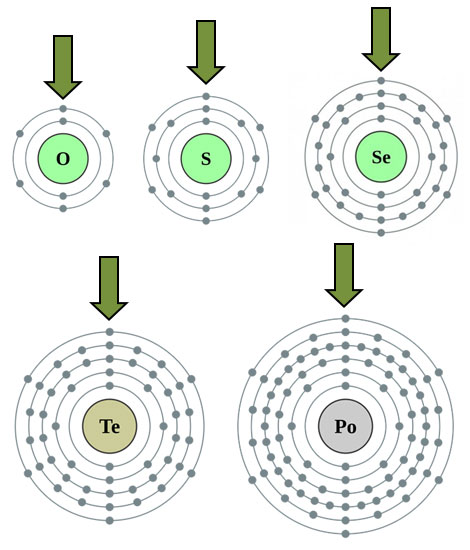
Source: Alkali Metals, Theodore W. Gray, Periodic Table.com
Now, look at the electron configuration for each of the alkali metals.
Element | Electron Configuration |
Oxygen | 1s22s22p4 |
Sulfur |
1s22s22p63s23p4 |
Selenium |
1s22s22p63s23p63d104s24p4 |
Tellurium |
1s22s22p63s23p63d104s24p64d105s25p4 |
Polonium |
1s22s22p63s23p63d104s24p64d104f145s25p65d106s26p4 |
Notice that the outermost energy level of each element consists of an s sublevel with two electrons and a p sublevel with four electrons.
Element | Electron Configuration |
Oxygen | 1s22s22p4 |
Sulfur |
1s22s22p63s23p4 |
Selenium |
1s22s22p63s23p63d104s24p4 |
Tellurium |
1s22s22p63s23p63d104s24p64d105s25p4 |
Polonium |
s22s22p63s23p63d104s24p64d104f145s25p65d106s26p4 |
The electrons found in s and p orbitals of the outer most energy level are called valence electrons. These are the electrons that are involved in bonding because they are more loosely held to the nucleus, as they are further away from the nucleus than other electrons. Valence electrons give the elements their characteristics. Since all the elements in the same group have the same number of valence electrons, they have similar characteristics.
The alkali metals have one valence electron, and the elements in the oxygen family have six valence electrons.
Element | Electron Configuration |
Lithium | 1s22s1 |
Sodium | 1s22s22p63s1 |
Potassium | 1s22s22p63s23p64s1 |
Rubidium | 1s22s22p63s23p63d104s24p65s1 |
Cesium | 1s22s22p63s23p63d104s24p64d105s25p66s1 |
Francium | 1s22s22p63s23p3d104s24p64d104f145s25p65d106s26p67s1 |
Oxygen | 1s22s22p4 |
Sulfur |
1s22s22p63s23p4 |
Selenium |
1s22s22p63s23p63d104s24p4 |
Tellurium |
1s22s22p63s23p63d104s24p64d105s25p4 |
Polonium |
s22s22p63s23p63d104s24p64d104f145s25p65d106s26p4 |
You’ll often need to know how many valence electrons an element has. It would be quite time consuming to list the complete electron configuration of an element every time you needed to know how many valence electrons there were in a particular element. Luckily, the periodic table tells you this information.
Earlier, it was pointed out that the alkali metals were in group 1A of the periodic table. You just learned that they have one valence electron. You also saw that the oxygen family was in group 6A on the periodic table and had six valence electrons. This matching of the group number and the number of valence electrons is not a coincidence. The group number identifies the number of valence electrons. This is very useful information!
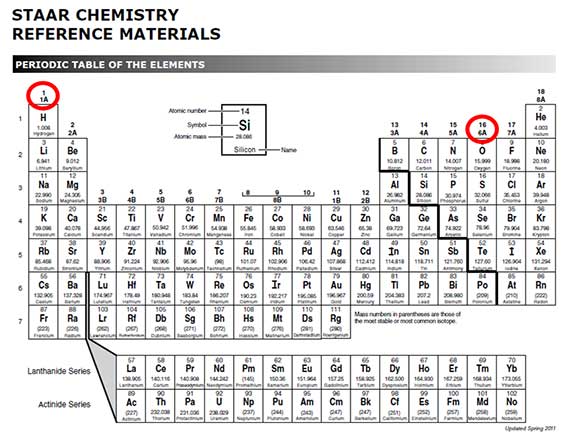
Boron is in group 3A.
Interactive popup. Assistance may be required.How many valence electrons does boron have?
3
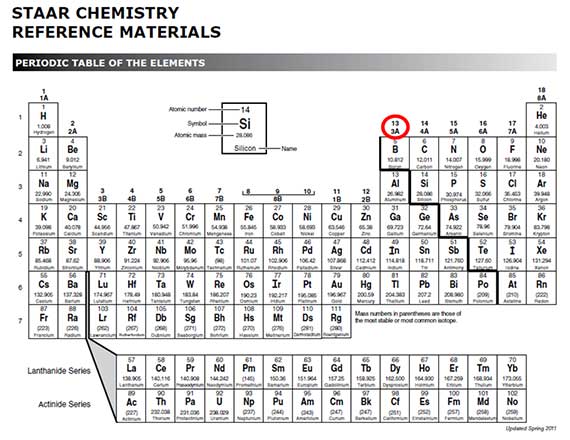
What about the transition metals?
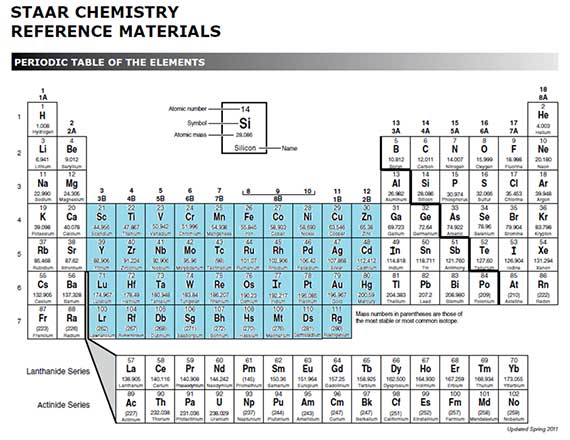
All transition metals have very similar properties. Listed below are some of the common properties of transition metals.
Transition metals have the following properties:
Transition metals are more complicated because they don't always use the same number of valence electrons in chemical reactions. For example, iron sometimes gives away two electrons, and other times it gives up three electrons.

Previous
Next