Well Done!
Reset
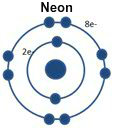
Why? Neon has its outer level completely filled with eight electrons.
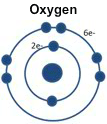
Oxygen's outer level is almost filled with six electrons.
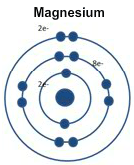
Magnesium only has two electrons in its outer level so it is lower in ionization energy than oxygen. But it has one less energy level than calcium. That makes it harder to lose its outer electrons.
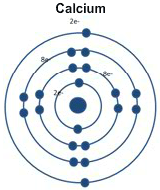
Calcium only has two in its outer energy level. Those two electrons are farther away from the nucleus than magnesium's outer electrons. That means it is easier for it to lose the outer electrons.
HIGHEST <--------------------------------------------> LOWEST