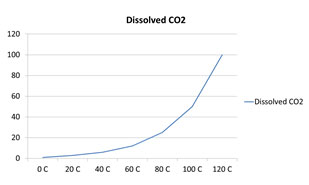
Incorrect. CO2 is a gas, so as the temperature increases, less CO2 is soluble.
At 70°C, which 1.0g sample of CuSO4 will dissolve the fastest in water?
A. Powdered CuSO4 with agitation
Correct! Powdered CuSO4 has the largest surface area and therefore will dissolve the quickest.
B. Large crystals of CuSO4 with agitation
Incorrect. Large crystals have a small surface area.
C. Powdered CuSO4 without agitation
Incorrect. Powdered CuSO4 has the largest surface area, but agitation is also needed to make it the fastest.
D. Large crystals of CuSO4 without agitation
Incorrect. Large crystals have a small surface area.
Before a lab investigation, students made predictions on the factors that affect the rate of solubility. Which set of conditions will result in the fastest rate of dissolving for a solid?
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | |
| Particle Size | Crystalline | Powder | Crystalline | Powder |
| Agitation | No Shaking | Shaking | No Shaking | Shaking |
| Temperature | 20°C | 20°C | 20°C | 20°C |
A. Trial 1
Incorrect. The low temperature and large surface area of the crystal will not allow it to dissolve as quickly as the other trials.
B. Trial 2
Incorrect. The low temperature makes this solution dissolve less quickly than Trial 4.
C. Trial 3
Incorrect. Although the temperature is the same as Trial 4, this trial uses a crystalline structure, which will dissolve more slowly than the powder because it has a smaller surface area exposed to the solvent.
D. Trial 4
Correct! The high temperature, agitation, and large surface of the powder will allow it to dissolve more quickly than others.
Temperatures in Texas can stay over 100°F for many days in the summer. This causes the temperature of the lakes and rivers to rise. Which of the following is the greatest danger to fish as water temperatures increase?
A. O2 molecules will increase in size.
Incorrect. Only the motion of molecules changes with temperature changes. The size of molecules does not change.
B. O2 solubility will decrease.
Correct!
C. O2 solubility will increase.
Incorrect. Increasing temperature causes gas solubility to decrease.
D. O2 molecules will form precipitates.
Incorrect. O2 molecules will not form precipitates.
Which of the following graphs correctly represents the solubility of CO2 in water?
A.

Incorrect. CO2 is a gas, so as the temperature increases, less CO2 is soluble.
Incorrect. CO2 is a gas, so as the temperature increases, less CO2 is soluble.
Correct!
C.
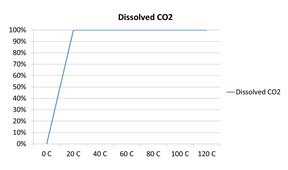
Incorrect. CO2 is a gas, so as the temperature increases, less CO2 is soluble.
D.
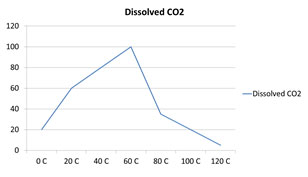
Incorrect. CO2 is a gas, so as the temperature increases, less CO2 is soluble.