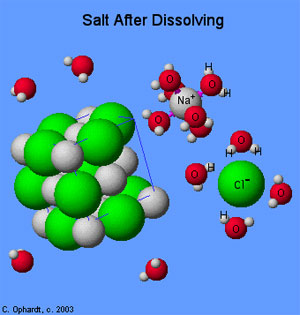
Study the diagram above. Using your knowledge of chemistry and evidence from the picture, determine why the water molecules orients themselves around the sodium and chlorine ions.
A. Water is the universal solvent and can dissolve any type of substance.
Incorrect. Water is the universal solvent, but this does not explain why it orients itself in a specific way around the sodium and chlorine atoms.
B. Water can form a hydrogen bond with the sodium ion to dissolve the crystal.
Incorrect. The hydrogen bonds in water are not responsible for dissolving the crystal.
C. Water is a non-polar molecule, which allows it to attract the sodium and chlorine ions.
Incorrect. Water is polar because the electrons are not equally shared between the hydrogen and oxygen atoms in the molecule.
D. Water is a polar molecule, which allows the hydrogen to bond with the chlorine ion and oxygen to bond with the sodium ion.
Correct! Water is polar, and the partial charges of hydrogen and oxygen are attracted to the opposite charges of the ions in the crystal.
Using your knowledge of water, determine which of the following substances would most likely be soluble when placed in a bucket of water.
A. Carbon dioxide, CO2 (non-polar)
Incorrect. Water is polar and only dissolves other polar or ionic substances.
B. Methanol, CH4 (non-polar)
Incorrect. Water is polar and only dissolves other polar or ionic substances.
C. Sulfuric acid, H2SO4 (polar)
Correct! Water is polar and will dissolve other polar or ionic substances.
D. Carbon tetrachloride, CCl4 (non-polar)
Incorrect. Water is polar and only dissolves other polar or ionic substances.
Which property of water is most responsible for the survival of fish during the winter?
A. Polar bonds
Incorrect. Water does have polar bonds, but it is not the reason fish are protected in the winter.
B. Hydrogen bonds
Correct! Hydrogen bonding is the reason solid H20 is less dense than liquid H2O and actually floats.
C. Dissociation of hydrogen and hydroxide ions
Incorrect. Water dissociation does not help fish survive in the winter.
D. Universal solvent
Incorrect. Water’s ability to dissolve many substances does help aquatic life survive, but not in the winter.
Water molecules tend to stick together (cohesion) and to other substances (adhesion), which allows plants to transport water from the roots to the top of trees.Which statement below best explains why cohesion happens in water molecules?
A. Water is polar, and the partial charges on the atoms are attracted to the partial or full charges of surrounding molecules.
Correct! Water polarity allows for cohesion and adhesion.
B. Water is covalently bonded so the electrons are shared between the elements, which allow the electrons to be shared between neighboring molecules.
Incorrect. Water is covalently bonded, but it keeps its electrons within the molecule. Cohesion and adhesion do not involve the sharing of electrons.
C. Water is an ionic compound that readily dissolves in the presence of other substances.
Incorrect. Water is covalent, not ionic.
D. Water is a diatomic molecule that would prefer to bond with other elements and not itself.
Incorrect. Water is a covalent compound and cohesion and adhesion does not involve breaking any bonds in a water molecule.