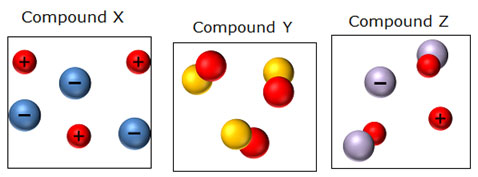
The more a compound dissociates in water, the better it conducts electricity. Aqueous solutions of three compounds are shown below.

Which of these can be considered a non-electrolyte?
A. X only
Incorrect. Dissociated ions are present.
B. Y only
Correct! No ions are present in this solution.
C. X and Y only
Incorrect. Compound X has ions present in solution.
D. Y and Z only
Incorrect. Compound Z has ions present in solution.
A solid substance was tested in the laboratory. The test results are listed below.
|
Based on these results, the solid substance could be —
A. Cu
Incorrect. Copper is a solid metal and will not dissociate in solution.
B. CuBr2
Correct! CuBr2 is a soluble ionic compound that will dissociate into ions.
C. CaCO3
Incorrect. CaCO3 is insoluble and will not dissociate into ions.
D. C6H12O6
Incorrect. Glucose is a covalent compound and will not dissociate into ions.
Which of the following is an electrolyte?
A. CH3OH
Incorrect. Methanol is a covalent compound and will not dissociate.
B. H2O
Incorrect. Water is a covalent compound and will not dissociate.
C. C6H12O6
Incorrect. Glucose is a covalent compound and will not dissociate.
D. KOH
Correct! Potassium hydroxide is an ionic compound and will dissociate into ions in solution.
Which of these salts has the highest conductivity?
A. NaNO3
Incorrect. NaNO3 will only dissociate into two ions.
B. MgCO3
Incorrect. MgCO3 is insoluble.
C. AgCl3
Correct! AgCl3 will dissociate into four ions.
D. CaCl2
Incorrect. CaCl2 will only dissociate into three ions.