Potassium hydroxide (KOH) is a strong base because it —
A. easily releases hydroxide ions
Correct! This is the definition of a strong base.
B. does not dissolve in water
Incorrect. There would be no release of hydroxide ions.
C. reacts to form salt crystals in water
Incorrect. There would be no release of hydroxide ions.
D. does not conduct an electric current
Incorrect. All bases conduct and electric current.
Which is a characteristic of a strong acid?
A. It has a pH greater than 7.
Incorrect. Acids have a pH less than 7.
B. It completely ionizes in a solution.
Correct! Strong acids completely dissociate.
C. It releases many hydroxide ions.
Incorrect. This is a characteristic of a strong base.
D. It reacts only with a strong base.
Incorrect. Acids will react with many things.
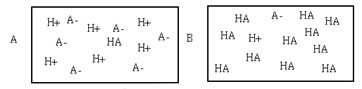
The images below both show 1L of 1M solutions of acid. Which of the following statements is true about these solutions?

A. Solution A is a weak acid and solution B is a strong acid.
Incorrect. Solution A is completely dissociated and must be a strong acid. Solution B has not completely dissociated and must be a weak acid.
B. Solutions A and B are both strong acids.
Incorrect. Solution B has not completely dissociated and must be a weak acid.
C. Solution A is a strong acid and solution B is weak acid.
Correct! Solution A is completely dissociated and must be a strong acid. Solution B has not completely dissociated and must be a weak acid.
D. Beakers A and B are both weak acids.
Incorrect. Beaker A is completely dissociated, and must be a strong acid.
Which of the following statements is NOT true?
A. 100 percent of [H+] ions dissociate in a strong acid.
Incorrect. This is the definition of a strong acid.
B. [OH-] ions are easily released from strong bases.
Incorrect. This is the Arrhenius definition of a strong base.
C. A 0.01M solution of hydrochloric acid can be categorized as a diluted strong acid.
Incorrect. HCL is a strong acid and 0.01M is a dilute solution.
D. It is impossible to have a concentrated weak acid.
Correct! It is possible to have a concentrated solution.