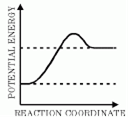

The reaction pictured in the potential energy diagram above is —
A. exothermic because it releases energy
Incorrect. The products have more energy than the reactants therefore energy was absorbed.
B. exothermic because it absorbs energy
Incorrect. Exothermic reactions release heat.
C. endothermic because is releases energy
Incorrect. Endothermic does not release energy.
D. endothermic because it absorbs energy
Correct! The products have more energy than the reactants therefore energy was absorbed.
What is the difference between the potential energy of the products and the potential energy of the reactants called in a chemical reaction?
A. Free energy ΔG
Incorrect. Free energy is the sum of its enthalpy, plus the product of the temperature and entropy of the system.
B. Activation energy Ea
Incorrect. Activation energy is the minimum energy that must be put into a chemical system in order for a chemical reaction to occur.
C. Heat of reaction ΔH
Correct!
D. Heat of fusion ΔHf
Incorrect. Heat of fusion is the energy needed to change one gram of a substance from the solid state to the liquid state without changing its temperature.
A gas at 25°C has a volume of 3.80 liters at a pressure of 1.00 atm. What will be its volume when the pressure is changed to 2.60 atm?
A. 1.46 L
Correct!
B. 9.88 L
Incorrect. Check your calculation. Use Boyle’s law.
C. 1.6 L
Incorrect. Check your calculation. Use Boyle’s law.
D. 0.5 L
Incorrect. Check your calculation. Use Boyle’s law.
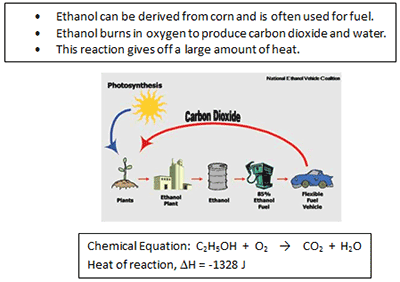
According to the information above, when ethanol reacts with oxygen, the process is___________.
A. endothermic
Incorrect. The ΔH for an endothermic reaction is positive.
B. exothermic
Correct! The ΔH is negative and the reaction gives off a large amount of energy.
C. neutral
Incorrect. There is no mention of the pH.
D. physical change
Incorrect. New products were formed; this is a chemical reaction.
The specific heat of copper is almost 0.4 Joules/ gram °C. How much heat must be applied to change the temperature of a 10-gram sample of copper from 30°C to 70°C?
A. 160J
Correct!
B. 680J
Incorrect. Use q=mcΔt to calculate the answer.
C. 560J
Incorrect. Use q=mcΔt to calculate the answer.
D. 400J
Incorrect. Use q=mcΔt to calculate the answer.
Given the reaction: Fe + S → FeS + energy
Which statement about this reaction is true?
A. It is endothermic.
Incorrect. Energy is a reactant in an endothermic reaction.
B. The potential energy of the reactants is lower than the potential energy of the product.
Incorrect. Energy was released therefore the reactants have more energy than the products.
C. It is exothermic.
Correct!
D. The potential energy of the reactants is the same as the potential energy of the product.
Incorrect. Energy was released from the reactants.
A beaker of cold water is dumped into a larger beaker of hot water. The water is mixed and then left alone for a long period of time. After being left alone, the temperature of the water is found to —
A. be hotter at the bottom of the beaker
Incorrect. The hot water will transfer heat to the cold water until the temperature reaches equilibrium.
B. fluctuate at all positions in the beaker
Incorrect. The hot water will transfer heat to the cold water until the temperature reaches equilibrium.
C. be the same throughout the beaker
Correct! The hot water will transfer heat to the cold water until the temperature reaches equilibrium.
D. randomly vary from one end to the other in the container
Incorrect. The hot water will transfer heat to the cold water until the temperature reaches equilibrium.
A 5.0 sample at 25°C and 3.00 atm of pressure contains 0.5 moles of gas. If an additional 0.75 moles of gas at the same pressure and temperature are added, what will be the final volume of the gas?
A. 2.0 L
Incorrect. Use PV=nRT to calculate.
B. 9.0 L
Incorrect. Use PV=nRT to calculate.
C. 5.8 L
Incorrect. Use PV=nRT to calculate.
D. 12.5 L
Correct!
When a light bulb is turned on, energy converts from one form to another. Which of the following best describes this change?
A. Electrical energy to light energy
Correct!
B. Sound energy to light energy
Incorrect. Electrical energy powers a light bulb.
C. Nuclear energy to light energy
Incorrect. Electrical energy powers a light bulb.
D. Magnetic energy to light energy
Incorrect. Electrical energy powers a light bulb.
The pressure of a mixture of nitrogen, carbon dioxide, and oxygen is 250 kPa. What is the partial pressure of oxygen if the partial pressure of nitrogen is 100 kPa and the partial pressure of carbon dioxide is 56 kPa?
A. 94 kPa
Correct!
B. 100 kPa
Incorrect. Use Dalton’s law of partial pressure to solve.
C. 294 kPa
Incorrect. Use Dalton’s law of partial pressure to solve.
D. 150 kPa
Incorrect. Use Dalton’s law of partial pressure to solve.
Which picture best represents a gas in a closed container?
A. 
Correct! Particles of gas will fill the entire container and be in constant random motion.
B. 
Incorrect. Particles of gas will fill the entire container and be in constant random motion.
C.

Incorrect. Particles of gas will fill the entire container and be in constant random motion.
D.
Incorrect. Particles of gas will fill the entire container and be in constant random motion.
When 1 gram of H2O(g) changes to 1 gram of H2O(l), the entropy of the system —
A. decreases
Correct! The water molecules are moving more slowly (more orderly) due to lower energy.
B. increases
Incorrect. The entropy is lower for a liquid than a gas.
C. need more information
Incorrect. The entropy is lower for a liquid than a gas. The water molecules are moving slower (more orderly) due to lower energy.
D. remains the same
Incorrect. The water molecules are moving slower (more orderly) due to lower energy.
When eating a bag of chips, the chemical energy stored in the chips —
A. increases
Incorrect. Energy in a system is conserved.
B. decreases
Incorrect. Energy in a system is conserved.
C. changes form
Correct! Energy is converted from chemical to mechanical or thermal energy.
D. is destroyed
Incorrect. Energy is always conserved.
If 4 moles of a gas are at a pressure of 5.6 atm and a volume of 12 liters, what is the temperature of the gas?
A. 100 K
Incorrect. This is the volume of one mole of gas.
B. 295 K
Incorrect. Use PV=nRT.
C. 205 K
Correct! You used PV=nRT.
D. 450 K
Incorrect. Use PV=nRT.
Stacey decided to cook pasta for dinner. She fills up her pot with water. The initial temperature of the water is 20°C, and the final temperature of the water is 99oC. The specific heat of water is 4.184 Joules/ gram°C. Determine the heat absorbed by 105.0 grams of H2O when it is heated to cook the pasta.
A. 50,654.78 J
Incorrect. Use Q=mc∆t.
B. 5.56 J
Incorrect. Use Q=mc∆t.
C. 1,000.00 J
Incorrect. Use Q=mc∆t.
D. 34,706.28 J
Correct! You used Q=mc∆t.
When the enthalpy(ΔH) of a reaction is greater than zero, the reaction is —
A. endothermic
Correct! A positive ∆H is endothermic.
B. exothermic
Incorrect. An exothermic reaction would have a negative ∆H.
C. nuclear
Incorrect. A nuclear equation will release heat and be exothermic.
D. no reaction
Incorrect. A reaction has occurred in this example.
What is the volume of 4.00 moles of N2 at STP?
A. 89.6 L
Correct! You used PV=nRT.
B. 22.4 L
Incorrect. This is the volume of one mole of any gas.
C. 11.2 L
Incorrect. Use PV=nRT.
D. 44.8 L
Incorrect. Use PV=nRT.
What type of energy is associated with the random motion of atoms and molecules?
A. Chemical energy
Incorrect. Chemical energy is stored in chemical bonds.
B. Endothermic
Incorrect. Endothermic is not a type of energy.
C. Thermal energy
Correct! The more movement of molecules, the more thermal energy.
D. Mechanical energy
Incorrect. Mechanical energy is the movement of objects.
71.5 grams of aluminum metal is heated from 20°C to 50°C in order to increase its malleability. The specific heat of aluminum is 0.900 J/g°C. What amount of heat is absorbed by the aluminum?
A. -368.7 J
Incorrect. The block is absorbing heat so ∆H should be positive.
B. +368.7 J
Incorrect. Use Q=mc∆T.
C. + 1,930.5 J
Correct! You used Q=mc∆t.
D. -1,930.5 J
Incorrect. The block is absorbing heat so ∆H should be positive.
Is the reaction in problem 20 an exothermic or endothermic process?
A. Endothermic
Correct! Absorbing heat is endothermic.
B. Exothermic
Incorrect. Absorbing heat is endothermic.
C. Both endothermic and exothermic
Incorrect. Heat is absorbed, so this process is only endothermic.
D. More information is needed
Incorrect. The only necessary information is that heat is absorbed.
What is the volume occupied by 12.0 grams of a gas at STP its molecular mass is 44.0?
A. 22.4 L
Incorrect. This is the volume of one mole of gas.
B. 4.4 L
Incorrect. Use PV=nRT.
C. 6.1 L
Correct! You used PV=nRT.
D. 5.5 L
Incorrect. Use PV=nRT.
How many calories of heat energy are absorbed when 100g of water is heated from 20 degrees Celsiuis to 30 degrees Celsius? (The specific heat of water is 1 calorie.)
A. 1000 cal
Correct! You used Q=mc∆t.
B. 10,000 cal
Incorrect. Use Q=mc∆t.
C. 100,000 cal
Incorrect. Use Q=mc∆t.
D. 100 cal
Incorrect. Use Q=mc∆t.
If the volume of an ideal gas is held constant when the temperature (K) is increased, the pressure will —
A. vary inversely
Incorrect. Pressure and temperature have a direct relationship.
B. decrease
Incorrect. Pressure and temperature have a direct relationship.
C. be unchanged
Incorrect. Pressure is not held constant in this system.
D. increase proportionately
Correct! Increased temperature results in more collisions and therefore an increase in pressure.
A jar containing molecules of gas A and a separate jar containing molecules of gas B are both at the same temperature. Gases A and B must have equal —
A. masses
Incorrect. Different gases will have different masses at the same temperature.
B. volumes
Incorrect. Different gases will take up different volumes.
C. pressures
Incorrect. Different gases may have different pressures.
D. average kinetic energy
Correct! Temperature is a measure of kinetic energy.
A 600 mL sample of nitrogen was heated from 17°C to 77°C at a constant pressure. What was the final volume?
A. 624.13 mL
Incorrect. Check your calculation. Use Charles’s law to calculate.
B. 724.14 mL
Correct!
C. 600.000 mL
Incorrect. Check your calculation. Use Charles’s law to calculate.
D. 497.14 mL
Incorrect. Check your calculation. Use Charles’s law to calculate.