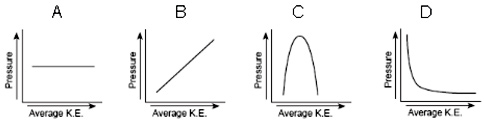
The relationship between the pressure of a gas and average kinetic energy at a fixed volume can best be shown by which of the following graphs?

A.
Incorrect. This graph shows no change in pressure as average kinetic energy increases. From KMT theory we know that pressure will increase in a fixed volume as the average kinetic energy increases.
B.
Correct! As average kinetic energy increases pressure will increase in a fixed volume.
C.
Incorrect. This graph shows an increase in pressure and then a decrease in pressure as average kinetic energy increases. From KMT theory we know that pressure will increase in a fixed volume as the average kinetic energy increases.
D.
Incorrect. This graph shows a decrease in pressure as average kinetic energy increases. From KMT theory we know that pressure will increase in a fixed volume as the average kinetic energy increases.
A gas is placed into the container shown below. At a constant temperature the volume of the gas is decreased which causes the pressure to increase because the gas particles do what ?
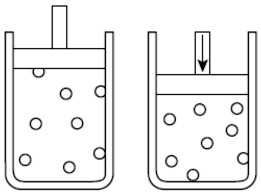
A. Expand to fill the space
Incorrect. At a constant temperature pressure and volume are inversely related (Boyle’s Law) so the particles get closer together as the volume is decreased.
B. Increase in velocity
Incorrect. Energy is conserved so at constant temperature the average kinetic energy of the particles will remain the same, even as volume is decreased.
C. Shrink to fit the container
Incorrect. The size of the gas molecules do not change as the volume is decreased.
D. Collide more often with the sides of the container
Correct! At constant temperature the gas particles will collide more frequently with the sides of the container as volume is decreased which causes the pressure to increase.
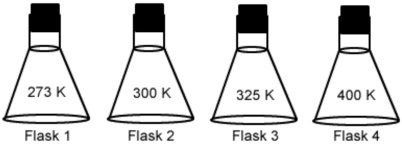
Four flasks are filled with 500mL of oxygen gas at 1atm. If each flask is allowed to heat up to the temperature shown on the flask, which one would most likely have the greatest pressure?
A. Flask 1
Incorrect. Gas molecules increase in kinetic energy as temperature increases, this flask does not have the highest kinetic energy.
B. Flask 2
Incorrect. As molecules increase in kinetic energy as temperature increases, this flask does not have the highest kinetic energy.
C. Flask 3
Incorrect . Gas molecules increase in kinetic energy as temperature increases, this flask does not have the highest kinetic energy.
D. Flask 4
Correct! Gas molecules increase in kinetic energy as temperature increases, this flask has the highest kinetic energy and therefore the greatest pressure.
Why does the pressure inside a container of gas increase if more gas is added to the container?
A. Because there is a corresponding increase in the number of particles striking an area of the wall of the container per unit time
Correct! As the number of particles increases in a container there are more collisions of the particles with the sides of the container which increases pressure.
B. Because there is a corresponding increase in the temperature
Incorrect. Simply adding more gas to a container does not increase the temperature.
C. Because there is a corresponding decrease in volume
Incorrect. Simply adding more gas to a container does not decrease the volume.
D. Because there is a corresponding increase in the force of the collisions between the particles and the walls of the container
Incorrect. Adding more gas to a container does not increase the force of the collisions but increases the number of collisions over time.