Dry air is composed of approximately 78% nitrogen, 20% oxygen and about 2% of other gasses at STP (0°C and 1atm). What is the partial pressure due to the nitrogen at STP?
A. 1atm
Incorrect. 1atm is the total pressure of all of the gasses.
B. 0.2atm
Incorrect. 0.2atm is the partial pressure due to oxygen (1atm × 20%).
C. 0.78atm
Correct! 0.78atm is the partial pressure due to nitrogen (1atm × 78%).
D. 0.98atm
Incorrect. 0.98atm is the partial pressure due to nitrogen + oxygen.
Three separate flasks contain chlorine at a pressure of 123kPa, bromine at a pressure of 29.5kPa, and fluorine at a pressure of 47.9kPa. If the three gasses were mixed together in a fixed volume flask of the same size, what would the total pressure be inside this flask?
A. 200.4 kPa
Correct! This is the total pressure inside the container (123kPa chlorine + 29.5kPa bromine + 47.9kP fluorine).
B. 123 kPa
Incorrect. This is the partial pressure due to chlorine only.
C. 77.4 kPa
Incorrect. This is the partial pressure of bromine + fluorine (29.5kPa + 47.9kPa).
D. 45.6 kPa
Incorrect. This is the partial pressure of chlorine – the partial pressure of bromine + fluorine (123kPa – (29.5kPa + 47.9kPa)

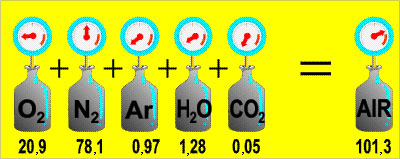
The graphic above shows the relative amounts of gasses found in air at sea level. A mountain climber starts climbing Mt. Everest and at the top the air pressure is only 37.5% of what the air pressure is at sea level. Calculate the partial pressure of oxygen at the top of Mt. Everest.
A. 20.9 kpa
Incorrect. This is the partial pressure of oxygen at sea level.
B. 7.8 kPa
Correct! Air pressure on the top of Mt. Everest is only 37.5% of the pressure at sea level, so the partial pressure of oxygen will be.
C. 80 kPa
Incorrect. This is total pressure at sea level – partial pressure of oxygen at sea level (101.3kPa – 20.9kPa).
D. 101.3 kPa
Incorrect. This is the total air pressure at sea level.
A mixture of neon, argon, and krypton gases exerts a total pressure of 2.39 atm. The partial pressure of the neon alone is 1.14 atm and the partial pressure of the argon alone is 0.75 atm. What is the partial pressure of the krypton?
A. 4.28 atm
Incorrect. This is the total pressure + the partial pressure of neon and argon (2.39atm + 1.14atm + 0.75atm).
B. 2.78 atm
Incorrect. This is the total pressure + the partial pressure due to neon – the partial pressure due to argon (2.39atm + 1.14atm – 0.75atm).
C. 1.25 atm
Incorrect. This is the total pressure – the partial pressure of neon (2.39atm – 1.14atm).
D. 0.5 atm
Correct! This is the total pressure – the partial pressures of neon and argon (2.39atm – 1.14atm – 0.75atm).