

The National Oceanic and Atmospheric Association (NOAA) is sending up several weather balloons to collect information about the upper atmosphere. In order to track their data, they must keep records of different changes in the balloons. If a balloon containing 105 liters of helium at standard temperature and pressure is sent up to the upper atmosphere and sends back readings of -900C and a pressure of 0.011 atm what is the new volume of the weather balloon?
A. The volume is 13.66 L
Incorrect. Check your decimals.
B. The volume is 1366 L
Correct! You applied the gas laws correctly.
C. The volume is 671 L.
Incorrect. Make sure to convert Celsius to Kelvin.
D. The volume is 136 L.
Incorrect. Check the number used for pressure.
A company that supplies different types of gases to other companies is sending a cylinder of nitrogen to a factory that makes ammonia. The standard container the gas supply company uses is a rigid steel container has a volume of 20 L and a pressure of 2.00 X 104 kPa that is kept at a temperature of 28oC. The ammonia factory needs to know how many moles are in this container so that they can order the proper number of containers. What is the number of moles in the containers?
A. There are 160 moles of nitrogen gas.
Correct! You applied the gas laws correctly.
B. There are 1720 moles of nitrogen gas.
Incorrect. Check temperature conversion.
C. There are 16185 moles of nitrogen gas.
Incorrect. Check “R” constant.
D. There are 16 moles of nitrogen gas.
Incorrect. Check the number used for pressure.
Using the information below, what is the pressure of the nitrogen gas in the cylinder?

A. The pressure is 0.821 atm.
Incorrect. Check temperature conversion.
B. The pressure is 8084 atm.
Incorrect. Check temperature conversion.
C. The pressure is 97.8 atm.
Incorrect. Check initial volume.
D. The pressure is 9.70 atm.
Correct! You applied the gas laws correctly.
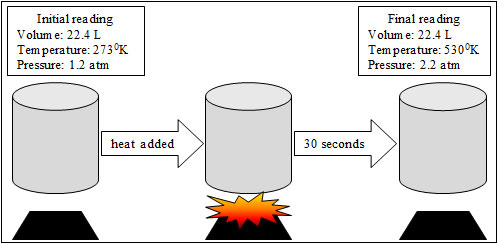
Students were completing a simulation online in which they were looking at temperature and pressure as related to the ideal gas law. The students set their initial readings at standard temperature and pressure (STP) for an ideal gas and then added heat and took a final reading after 30 seconds. Using the image above, which graph statement best represents what the students observed during the investigation?
A. If temperature increases, then pressure increases
Correct! You applied the gas laws correctly.
B. If temperature stays the same, pressure increases
Incorrect. Look at the two temperature readings.
C. If temperature increases, pressure is the same
Incorrect. Look at the two pressure readings.
D. If temperature increases, then pressure decreases
Incorrect. Look at the two pressure readings.