A balloon has a volume of 1.20 L at 24.0°C. The balloon is heated to 48.0°C. Calculate the new volume of the balloon.
A. 1.30 L Incorrect.
1st convert temperatures to Kelvin by adding 273
2nd plug values in Charles’ Law V1T2 = V2T1
(1.2L)(321K) = (V2)(297K)
3rd solve for the unknown
V2 = 1.30L
B. 1.70 L Incorrect.
1st convert temperatures to Kelvin by adding 273
2nd plug values in Charles’ Law V1T2 = V2T1
(1.2L)(321K) = (V2)(297K)
3rd solve for the unknown
V2 = 1.30L
C. 2.10 L Correct!
1st convert temperatures to Kelvin by adding 273
2nd plug values in Charles’ Law V1T2 = V2T1
(1.2L)(321K) = (V2)(297K)
3rd solve for the unknown
V2 = 1.30L
D. 2.40 L Incorrect.
1st convert temperatures to Kelvin by adding 273
2nd plug values in Charles’ Law V1T2 = V2T1
(1.2L)(321K) = (V2)(297K)
3rd solve for the unknown
V2 = 1.30L
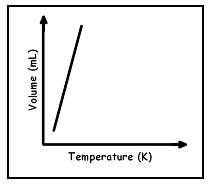
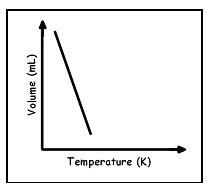
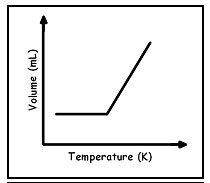
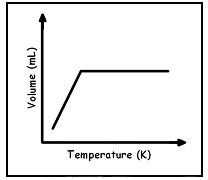
Which graph best shows the relationship between the volume of a gas and it's temperature as the gas pressure remains constant?




A.
Correct! As the temperature increases, volume increases according to Charles’ Law.
B.
Incorrect. As the temperature increases volume does not decrease it should increase.
C.
Incorrect. As the temperature increases the volume does not hold constant and then increase. As temperature increases, the volume should increase as well.
D.
Incorrect. As temperature increases volume does increase but volume doesn’t become constant at a certain temperature it would continue to increase.
Charles’ Law states that if a given quantity of gas is held at a constant pressure, then its volume is directly proportional to the absolute temperature. This law explains why —
A. the pressure of a gas increases when volume decreases
Incorrect. Pressure is held constant in Charles’ Law not increased.
B. solids require heat in order to change into gases
Incorrect. The gas laws only deal with gases not solids or liquids.
C. a gas-filled balloon expands when it is heated
Correct! If a gas is heated then it’s temperature is increasing which means the volume should also increase.
D. some gases only react with each other at high temperatures
Incorrect. This gas law does not tell us anything about the reactivity of a gas.
The two balloons below were filled with an identical number of moles of gas. Which of the following best explains why balloon B is larger than balloon A?
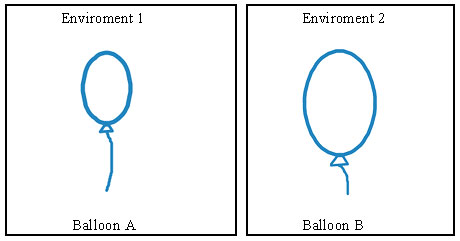
A. The gas in balloon B is cooler.
Incorrect. This would cause the balloon B to be smaller.
B. The gas in balloon A is under less pressure.
Incorrect. This would cause balloon A to be larger.
C. The gas in balloon A is warmer.
Incorrect. Heating balloon A would cause it to be larger.
D. The gas in balloon B is warmer.
Correct! Heating balloon B would cause it to be larger.